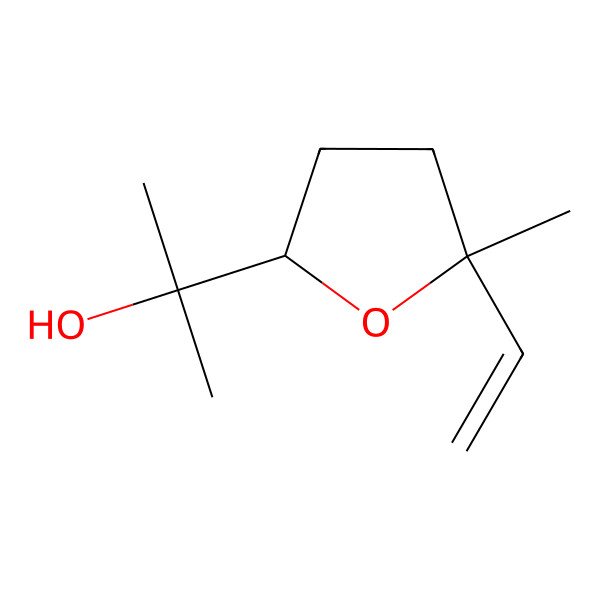
| Linalyl oxide |
| Epoxylinalool |
| 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol |
| linalooloxide |
| Linolool oxide |
| 2-(2-Hydroxy-2-propyl)-5-methyl-5-vinyltetrahydrofuran |
| EINECS 262-038-6 |
| 2-Methyl-2-vinyl-5-(1-hydroxy-1-methylethyl)tetrahydrofuran |
| 5-(1-Hydroxy-1-methylethyl)-2-methyl-2-vinyltetrahydrofuran |
| BRN 0117527 |
| 2-(Tetrahydro-5-methyl-5-vinyl-2-furyl)propan-2-ol |
| 2,6-Dimethyl-3,6-oxido-7-oxten-2-ol |
| 2-Methyl-2-vinyl-5-(2-hydroxy-2-propyl)tetrahydrofuran |
| alpha,alpha,5-Trimethyl-5-vinyltetrahydrofurfuryl alcohol |
| (Z)-Linalool oxide (furanoid) |
| 5-Ethenyltetrahydro-alpha,alpha,5-trimethyl-2-furanmethanol |
| 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl- |
| 2-(5-Methyl-5-vinyltetrahydro-1-furyl)-2-propanol |
| 2-(5-Methyl-5-vinyltetrahydrofuran-2-yl)propan-2-ol |
| 2-Furanmethanol, 5-ethenyltetrahydro-alpha,alpha,5-trimethyl- |
| Linalool oxide, (Z)- |
| 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- |
| 5989-33-3 |
| 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, (2R,5S)-rel- |
| 5-17-03-00253 (Beilstein Handbook Reference) |
| Linalool oxide B |
| cis-Linalyl Oxide |
| Furfuryl alcohol, tetrahydro-.alpha.,.alpha.,5-trimethyl-5-vinyl- |
| (Z)-Linalool oxide B |
| cis-Furan linalool oxide |
| UNII-2U90CGE5DT |
| Tetrahydro-alpha,alpha,5-trimethyl-5-vinylfuran-2-methanol |
| Linalool oxide I (cis, furanoid) |
| EINECS 227-814-0 |
| trans-5-Ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-2-furanmethanol |
| 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, trans- |
| 2-Furanmethanol, 5-ethenyltetrahydro-alpha,alpha,5-trimethyl-, (2R,5R)-rel- |
| Furan linalool oxide |
| Linalool oxide, cis |
| Linalool 3,6-oxide |
| cis-Linalol furanoxide |
| cis-Linalool furan oxide |
| cis-Linalool oxide furan |
| (Z)-Linalool furanoxide |
| cis-Furanic linalool oxid |
| cis-Linalool-3,6-oxide |
| linalool oxide (furanoid) |
| Z-Furanoid linalool oxide |
| Z-linalool oxide (furan) |
| (Z)-Furan linalool oxide |
| 2-(5-Methyl-5-vinyltetrahydro-2-furanyl)-2-propanol |
| Linalool oxide, cis-furanoid |
| cis-Linalyl oxide (furanoid) |
| 2-Furanmethanol, 5-ethenyltetrahydro-?,?,5-trimethyl-, trans- |
| 68780-91-6 |
| cis-5-Ethenyltetrahydro-alpha,alpha,5-trimethyl-2-furanmethanol |
| cis-linalool oxide (furanoid) |
| Linalool oxide (cis-furanoid) |
| SCHEMBL295274 |
| (Z)-Linalool oxide, furanoid |
| cis-2-Methyl-2-vinyl-5-(1-hydroxy-1-methylethyl)tetrahydrofuran |
| tetrahydro - a,a,5 - trimethyl - 5 - vinylfuran - 2 - methanol |
| (Z)-Linalool oxyde (furanoid) |
| 2-(5-ETHENYL-5-METHYL-OXOLAN-2-YL)PROPAN-2-OL |
| CHEBI:88534 |
| cis-Linalool oxide (furan type) |
| FEMA 3746 |
| cis-Linalol oxide (furan isomer) |
| DTXSID60863673 |
| 2-Furanmethanol, 5-ethenyltetrahydro-alpha,alpha,5-trimethyl-, (2R,5S)-rel- |
| Furfuryl alcohol, tetrahydro-.alpha.,.alpha.,5-trimethyl-5-vinyl-, (2R,5R)-(-)- |
| 2-Furanmethanol, 5-ethenyltetrahydro-?,?,5-trimethyl-, cis- |
| cis-Linalool oxide (furanyl ring) |
| Linalool oxide B ((Z)-furanoid) |
| Linalool oxide, natural, >=95% |
| cis-Linalool oxide (furanoid form) |
| NSC93936 |
| MFCD00053543 |
| NSC 93936 |
| NSC-93936 |
| 2-Furanmethanol,.alpha.,5-trimethyl- |
| AKOS040759301 |
| LS-2881 |
| 2,6-Dimethyl-3,6-oxido-7-octen-2-ol |
| L0143 |
| D91255 |
| EN300-176284 |
| 2-(5-Methyl-5-vinyltetrahydro-2-furyl)-2-propanol |
| Q27160424 |
| 2-(5-Methyl-5-vinyltetrahydro-2-furanyl)-2-propanol # |
| 2-Furanmethanol, 5-ethenyltetrahydro-?,?,5-trimethyl- |
| 5-Ethenyltetrahydro-a,a,5-trimethyl-2-furanmethanol, 9CI |
| 2-((2R,5S)-5-Methyl-5-vinyltetrahydrofuran-2-yl)propan-2-ol |
| Tetrahydro-2-methyl-5-(1-hydroxy-1-methylethyl)-2-vinylfuran |
| 5-Ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-cis-2-furanmethanol |
| cis-5-Ethenyltetrahydro-.alpha.,.alpha.,5-Trimethyl-2-furanmethanol |
| Z-2-Ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-furanmethanol |
| cis - a,a,5 - trimethyl - 5 - vinyltetrahydrofuran - 2 - methanol |
| Furfuryl alcohol, tetrahydro-.alpha.,.alpha.,5-trimethyl-5-vinyl-, cis- |
| 2 - (tetrahydro - 5 - methyl - 5 - vinyl - 2 - furyl) propan - 2 - ol |
|
There are more than 10 synonyms. If you wish to see them all click here.
|