| Helixin |
| Sapindoside A |
| Kalopanaxsaponin A |
| Hederoside C |
| Tauroside E |
| 27013-91-8 |
| Koronaroside A |
| Prosapogenin CP3b |
| Glycoside L-E1 |
| Akeboside Stc |
| Pulsatilla saponin A |
| Nepalin 2 |
| .alpha.-Hederin |
| Akebia saponin PD |
| Kizuta saponin K6 |
| alpha-Hederine |
| Helixin (VAN) |
| alpha-Hederin hydrate |
| alpha-Hederine [French] |
| EINECS 248-166-5 |
| NSC 106553 |
| BRN 0076156 |
| UNII-4H15F0GLV2 |
| 4H15F0GLV2 |
| CHEBI:69370 |
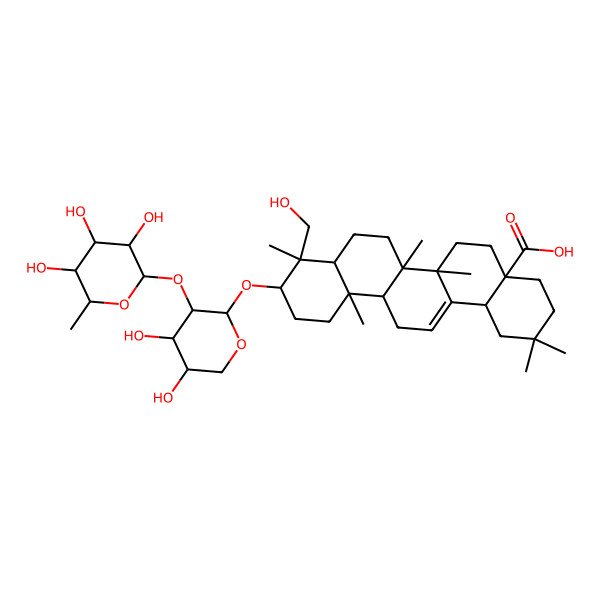
| C41H66O12 |
| 4-17-00-02560 (Beilstein Handbook Reference) |
| Hederin |
| Alpha Hederin |
| helixin (the saponin) |
| (4alpha)-3beta-((2-O-(6-Deoxy-alpha-L-mannopyranosyl)-alpha-L-arabinopyranosyl)oxy)-23-hydroxyolean-12-en-28-oic acid |
| (4aS,6aS,6bR,8aR,9R,10S,12aR,12bR,14bS)-10-(((2S,3R,4S,5S)-4,5-Dihydroxy-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid |
| Olean-12-en-28-oic acid, 3-((2-O-(6-deoxy-alpha-L-mannopyranosyl)-alpha-L-arabinopyranosyl)oxy)-23-hydroxy-, (3-beta,4-alpha)- |
| Rha-Ara-3beta-hederagenin |
| NSC106553 |
| Akebiasaponin PD |
| Kizutasaponin K6 |
| NSC-106553 |
| (4alpha)-3beta-[[2-O-(6-deoxy-alpha-L-mannopyranosyl)-alpha-L-arabinopyranosyl]oxy]-23-hydroxyolean-12-en-28-oic acid |
| kalopanax saponin A |
| ALFA-HEDERINA |
| .ALPHA.-HEDERIN [MI] |
| CHEMBL447565 |
| SCHEMBL3007691 |
| ALFA-HEDERINA [WHO-DD] |
| DTXSID80909168 |
| (4aS,6aR,6aS,6bR,8aR,9R,10S,12aR,14bS)-10-[(2S,3R,4S,5S)-4,5-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxy-tetrahydropyran-2-yl]oxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid |
| HY-N0255 |
| Olean-12-en-28-oic acid, 3-[[2-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.alpha.-L-arabinopyranosyl]oxy]-23-hydroxy-, (3.beta.,4.alpha.)- |
| s3914 |
| AKOS032961986 |
| CCG-270437 |
| CS-5690 |
| CS-O-10015 |
| (4aS,6aR,6aS,6bR,8aR,9R,10S,12aR,14bS)-10-[(2S,3R,4S,5S)-4,5-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid |
| AC-33961 |
| LS-98290 |
| C08954 |
| Q25105925 |
| 3-O-alpha-L-rhamnopyranosyl-(1->2)-alpha-L-arabinopyranosyl hederagenin |
| (4.ALPHA.)-3.BETA.-((2-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.ALPHA.- L-ARABINOPYRANOSYL)OXY)-23-HYDROXYOLEAN-12-EN-28-OIC ACID |
| (4ALPHA)-3BETA-((2-O-(6-DEOXY-ALPHA-L-MANNOPYRANOSYL)-ALPHA- L-ARABINOPYRANOSYL)OXY)-23-HYDROXYOLEAN-12-EN-28-OIC ACID |
| (4aS,6aR,6aS,6bR,8aR,9R,10S,12aR,14bS)-10-[(2S,3R,4S,5S)-4,5-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylicacid |
| (4aS,6aS,6bR,8aR,9R,10S,12aR,12bR,14bS)-10-(((2S,3R,4S,5S)-4,5-Dihydroxy-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylicacid |
| 3beta-{[2-O-(6-deoxy-alpha-L-mannopyranosyl)-alpha-L-arabinopyranosyl]oxy}-23-hydroxyolean-12-en-28-oic acid |
| OLEAN-12-EN-28-OIC ACID, 3-((2-O-(6-DEOXY-ALPHA-L- MANNOPYRANOSYL)-ALPHA-L-ARABINOPYRANOSYL)OXY)-23-HYDROXY-, (3-BETA,4-ALPHA)- |
| Olean-12-en-28-oic acid, 3-((2-O-(6-deoxy-alpha-L-mannopyranosyl)-alpha-L-arabinopyranosyl)oxy)-23-hydroxy-, (3beta,4alpha)- |
| Olean-12-en-28-oic acid, 3-[[2-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.alpha.-L-arabinopyranosyl]oxy]-23-hydroxy-, (3.beta., 4.alpha.) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|