| Linalool oxide A |
| Linalool oxide II |
| (E)-Linalool oxide A |
| trans-Linalool 3,6-oxide |
| E-Linalool oxide (furanoid) |
| Linalool A oxide |
| Linalool oxide 2 |
| trans-Linalool oxide (furanoid) |
| (E)-Furanoid linalool oxide |
| trans-Furanoid linalool oxide |
| trans-f-Linalool oxide |
| Linalool oxide, trans- |
| (E)-LINALOOL OXIDE |
| Epoxydihydrolinalool I |
| Linalool oxide, (E)- |
| Linalool oxide II (trans, furanoid) |
| trans-2-Methyl-2-vinyl-5-(1-hydroxy-1-methylethyl)tetrahydrofuran |
| trans-2-Vinyl-2-methyl-5-(1-hydroxy-1-methylethyl)tetrahydrofuran |
| UNII-986J3104O2 |
| EINECS 252-312-3 |
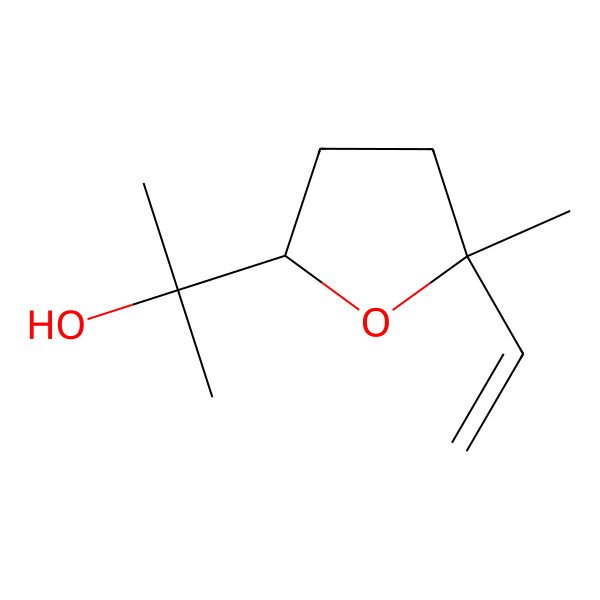
| 2-[(2S,5S)-5-ethenyl-5-methyloxolan-2-yl]propan-2-ol |
| 986J3104O2 |
| 11063-78-8 |
| 3,7-Dimethyl-1,6-octadien-3-ol epoxy deriv., trans- |
| 1,6-Octadien-3-ol, 3,7-dimethyl-, epoxy deriv., trans- |
| Furfuryl alcohol, tetrahydro-alpha,alpha,5-trimethyl-5-vinyl- |
| trans-alpha,alpha,5-Trimethyl-5-vinyltetrahydrofurfuryl alcohol |
| Linalool oxide, trans |
| t-Furan linalool oxide |
| (E)-Linalool furanoxide |
| trans-Linalool furanoxide |
| E-Furanoid linalool oxide |
| trans-Linalool oxide (f) |
| (E)-Furan linalool oxide |
| E-Linalool oxide (furan) |
| trans-Linalool oxide furan |
| 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, trans- |
| trans-Furanic linalool oxid |
| trans-Furanic linalool oxide |
| Linalool oxide (trans-THF) |
| trans-Linalool oxide (THF) |
| trans-Linalool furanoid oxide |
| (E)-Linalool oxide, furanoid |
| Linalool oxide, trans-furanoid |
| Linalool oxide A (trans-THF) |
| Tetrahydro-alpha,alpha,5-trimethyl-5-vinylfuran-2-methanol |
| trans-Linalool oxide (furan type) |
| trans-Linalool oxide (furanyl ring) |
| trans-Linalool oxide (furanoid form) |
| 2-Furanmethanol, 5-ethenyltetrahydro-alpha,alpha,5-trimethyl-, (2R,5R)-rel- |
| Linalool oxide trans |
| (+)-linalool oxide |
| 2-(5-Methyl-5-vinyltetrahydro-2-furanyl)-2-propanol |
| (+/-)-trans-Linalyl oxide |
| 2-((2R,5R)-5-Methyl-5-vinyltetrahydrofuran-2-yl)propan-2-ol |
| 2-(5-Methyl-5-vinyltetrahydro-2-furanyl)-2-propanol, trans- |
| 2-Furanmethanol, 5-ethenyltetrahydro-?,?,5-trimethyl-, trans- |
| tetrahydro - a,a,5 - trimethyl - 5 - vinylfuran - 2 - methanol |
| trans-.alpha.,.alpha.,5-Trimethyl-5-vinyltetrahydrofurfuryl alcohol |
| SCHEMBL1116444 |
| CHEMBL2252947 |
| BRHDDEIRQPDPMG-WCBMZHEXSA-N |
| CHEBI:177374 |
| Furfuryl alcohol, tetrahydro-.alpha.,.alpha.,5-trimethyl-5-vinyl-, (2R,5R)-(-)- |
| FEMA NO. 3746, TRANS- |
| (+/-)-LINALOOL OXIDE, (E)- |
| Q27272093 |
| 5-Ethenyltetrahydro-a,a,5-trimethyl-(2R,5R)-rel-2-Furanmethanol |
| 2-Furanmethanol, 5-ethenyltetrahydro-alpha,alpha,5-trimethyl-, trans- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|