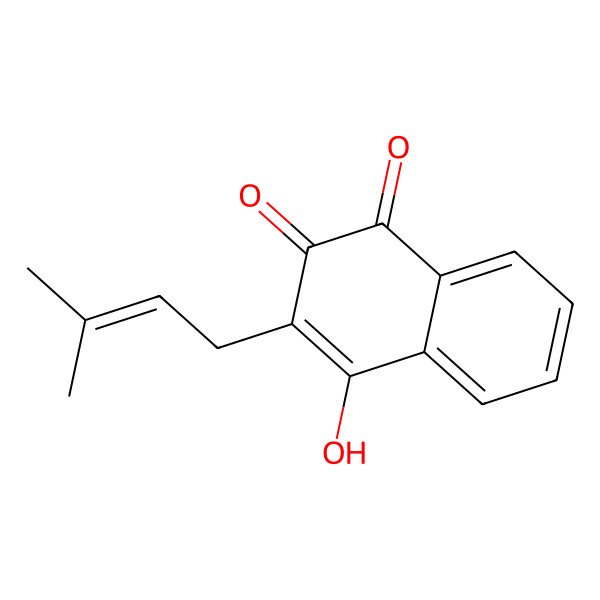
| 84-79-7 |
| Greenhartin |
| Tecomin |
| Bethabarra wood |
| Taiguic acid |
| Lapachol wood |
| Taigu wood |
| C.I. Natural Yellow 16 |
| Lapachic acid |
| IPE-tobacco wood |
| Greenharten |
| Surinam greenheart wood |
| NSC-11905 |
| C.I. 75490 |
| Zlut prirodni 16 |
| Tecomin (VAN) |
| NSC 11905 |
| 2-Hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone |
| Zlut prirodni 16 [Czech] |
| 4-hydroxy-3-(3-methylbut-2-enyl)naphthalene-1,2-dione |
| CCRIS 745 |
| 1,4-Naphthalenedione, 2-hydroxy-3-(3-methyl-2-butenyl)- |
| NSC 629756 |
| NSC11905 |
| EINECS 201-563-7 |
| NSC629756 |
| BRN 2051889 |
| 2-Hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthalenedione |
| 1,4-Naphthoquinone, 2-hydroxy-3-(3-methyl-2-butenyl)- |
| CHEBI:6377 |
| DTXSID6049430 |
| UNII-B221938VB6 |
| 2-Hydroxy-3-(3-methylbut-2-enyl)-1,4-naphthoquinone |
| B221938VB6 |
| NSC-629756 |
| 2-Hydroxy-3-(3-methyl-2-buten-1-yl)-1,4-naphthalenedione |
| 2-hydroxy-3-(3-methylbut-2-en-1-yl)naphthalene-1,4-dione |
| 4-08-00-02487 (Beilstein Handbook Reference) |
| Groenhartin |
| Lapachoic acid |
| Lapachol, 98% |
| Natural Yellow-?16 |
| 1,4-Naphthalenedione,2-hydroxy-3-(3-methyl-2-buten-1-yl)- |
| LAPACHOL [MI] |
| Spectrum2_001451 |
| Spectrum3_000768 |
| Spectrum5_001873 |
| bmse000989 |
| NCIMech_000076 |
| Oprea1_717083 |
| BSPBio_002416 |
| CHEMBL15193 |
| DivK1c_000594 |
| SCHEMBL157255 |
| SCHEMBL157256 |
| SPECTRUM1501204 |
| SPBio_001341 |
| 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphtho-quinone |
| DTXCID5029390 |
| HMS501N16 |
| KBio1_000594 |
| KBio3_001636 |
| NINDS_000594 |
| HMS1921B06 |
| HMS3869D03 |
| AMY40588 |
| BCP24022 |
| HY-N6961 |
| Tox21_202948 |
| CCG-35464 |
| MFCD00001679 |
| s5684 |
| AKOS015951424 |
| AKOS032948320 |
| AC-8971 |
| CI75490 |
| CS-W020951 |
| SDCCGMLS-0066666.P001 |
| CAS-84-79-7 |
| IDI1_000594 |
| NCGC00094931-01 |
| NCGC00094931-02 |
| NCGC00094931-03 |
| NCGC00094931-04 |
| NCGC00094931-06 |
| NCGC00260494-01 |
| WLN: L66 BV EVJ CQ D2UY1&1 |
| AS-35308 |
| CI 75490 |
| LS-95648 |
| NCI60_000457 |
| NCI60_000587 |
| PD087478 |
| 1, 2-hydroxy-3-(3-methyl-2-butenyl)- |
| FT-0649649 |
| A863979 |
| Cancer Chemother Rep (part 2) 4: 11 (1974) |
| Q739601 |
| SR-05000002489 |
| 2-Hydroxy-3-(3-methyl-2-butenyl)naphthoquinone # |
| SR-05000002489-1 |
| 2-hydroxy-3-(3-methylbut-2-enyl)-1,4-naphthoquinon |
| NSC-11905; NSC 11905; NSC11905 |
| 2-hydroxy-3-(3-methylbut-2-enyl)naphthalene-1,4-dione |
| 3-(3-Methyl-2-butenyl)-4-hydroxy-1,2-naphthoquinone |
| 4-hydroxy-3-(3-methylbut-2-en-1-yl)-1,2-dihydronaphthalene-1,2-dione |
|
There are more than 10 synonyms. If you wish to see them all click here.
|