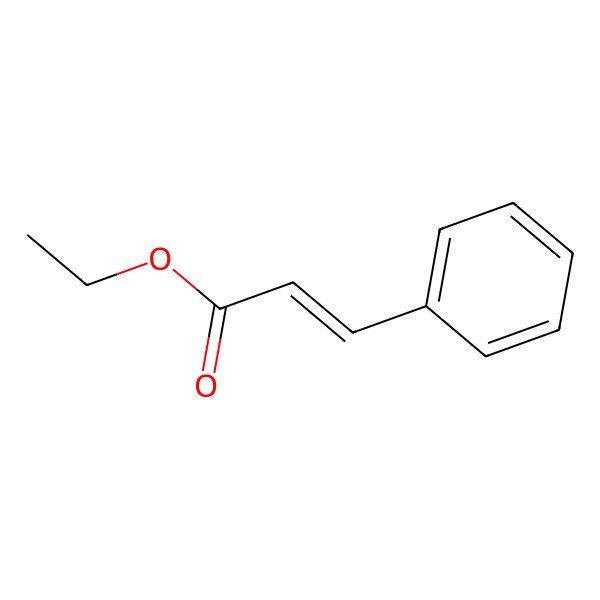
| 103-36-6 |
| Ethylcinnamate |
| Cinnamic acid ethyl ester |
| 4192-77-2 |
| Ethyl 3-phenylacrylate |
| Ethylcinnamoate |
| (E)-ethyl cinnamate |
| Cinnamic acid, ethyl ester |
| Ethyl 3-phenyl-2-propenoate |
| ethyl (E)-3-phenylprop-2-enoate |
| 2-Propenoic acid, 3-phenyl-, ethyl ester |
| Ethyl trans-cinnamate |
| Ethyl benzylideneacetate |
| Ethyl 3-phenylpropenoate |
| Ethyl beta-phenylacrylate |
| FEMA No. 2430 |
| Ethyl cinnamate (natural) |
| Ethyl (E)-cinnamate |
| SemaSORB 9827 |
| NSC 6773 |
| EINECS 203-104-6 |
| UNII-C023P3M5JJ |
| BRN 1238804 |
| C023P3M5JJ |
| ethyl-(E)-cinnamate,ethyl-trans-cinnamate |
| AI3-00667 |
| Ethyl 3-Phenylpropenate |
| NSC-6773 |
| ethyl (2E)-3-phenylprop-2-enoate |
| 3-Phenylpropenoic acid ethyl |
| Ethyl trans-Cinnamate-d5 |
| 3-Phenyl-2-propenoic acid, ethyl ester |
| CHEBI:4895 |
| DTXCID802520 |
| DTXSID9022520 |
| 2-Propenoic acid, 3-phenyl-, ethyl ester, (E)- |
| trans-Ethyl cinnamate |
| 3-Phenyl-2-propenoic acid ethyl ester |
| Ethyl 3-phenylprop-2-enoate |
| ethyl (2E)-3-phenylacrylate |
| ETHYL CINNAMATE (MART.) |
| ETHYL CINNAMATE [MART.] |
| cis-Ethyl Cinnamate (contains up to 10% Ethyl dihydrocinnamate |
| MFCD00009189 |
| Ethyl cinnamate, trans |
| 856765-68-9 |
| ethylcinnamat- |
| Ethyl (2E)-3-phenyl-2-propenoate |
| trans-ethylcinnamate |
| (E)-ethylcinnamate |
| ethyl-(e)-cinnamate |
| Ethyl Cinnamate Natural |
| Ethyl cinnamate, 99% |
| Benzeneacrylic acid ethyl |
| ethyl trans-cinnamic acid |
| Ethyl trans-3-Phenylacrylate |
| WLN: 2OV1U1R |
| 3-Phenyl-acrylicacidethylester |
| BIDD:ER0267 |
| ETHYL CINNAMATE [FCC] |
| SCHEMBL112445 |
| ETHYL CINNAMATE [FHFI] |
| ETHYL CINNAMATE [INCI] |
| CHEMBL318196 |
| trans-cinnamic acid ethyl ester |
| CINNAMIC ACID,ETHYL ESTER |
| Ethyl 3-Phenylpropenate, Natural |
| NSC6773 |
| Cinnamic acid, ethyl ester (6CI,7CI,8CI); 3-Phenyl-2-propenoic acid ethyl ester |
| DTXSID601017688 |
| 3-Phenyl-acrylic acid, ethyl ester |
| Cinnamic acid, ethyl ester, (E)- |
| HY-Y0121 |
| Ethyl cinnamate, analytical standard |
| Tox21_302638 |
| AC8020 |
| AKOS008947908 |
| Ethyl cinnamate, >=98%, FCC, FG |
| CINNAMIC ACID ETHYL ESTER [MI] |
| CS-W020629 |
| Ethyl (2E)-3-phenyl-2-propenoate # |
| LS-2716 |
| trans-3-Phenylacrylic Acid Ethyl Ester |
| (E)-3-Phenyl-acrylic acid ethyl ester |
| Ethyl cinnamate, natural, >=95%, FG |
| NCGC00256672-01 |
| AS-75482 |
| CAS-103-36-6 |
| CS-0356392 |
| EN300-49203 |
| C06359 |
| EN300-306003 |
| A896492 |
| Q204182 |
| 2-Propenoic acid, 3-phenyl-, ethyl ester, (2E)- |
| J-000950 |
| Z53834692 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|