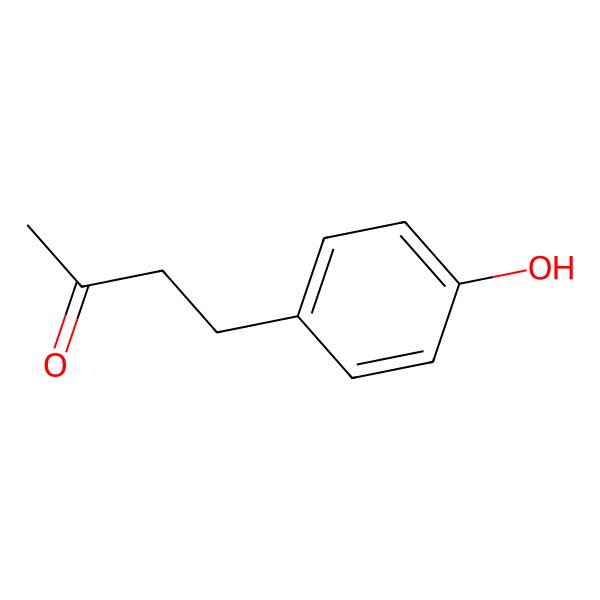
| Raspberry ketone |
| 5471-51-2 |
| 4-(4-Hydroxyphenyl)butan-2-one |
| Frambinone |
| Oxyphenalon |
| Rheosmin |
| 4-(p-Hydroxyphenyl)-2-butanone |
| Rasketone |
| 4-Hydroxybenzylacetone |
| p-Hydroxybenzyl acetone |
| 2-Butanone, 4-(4-hydroxyphenyl)- |
| 4-(3-Oxobutyl)phenol |
| 1-(p-Hydroxyphenyl)-3-butanone |
| 1-(4-Hydroxyphenyl)-3-butanone |
| rasberry ketone |
| (p-Hydroxybenzyl)acetone |
| 2-BUTANONE, 4-(p-HYDROXYPHENYL)- |
| FEMA No. 2588 |
| Hydroxyphenylbutanone, p- |
| p-hydroxyphenylbutan-2-one |
| NSC 26515 |
| RASPBERRYKETONE |
| EINECS 226-806-4 |
| UNII-7QY1MH15BG |
| BRN 0776080 |
| 7QY1MH15BG |
| 4-(p-Hydroxyphenyl)-2-butanone (natural) |
| AI3-31812 |
| CHEMBL105912 |
| DTXSID5044495 |
| CHEBI:68656 |
| HSDB 8163 |
| MFCD00002394 |
| NSC-26515 |
| 4-(4'-Hydroxyphenyl)-2-butanone-d5 |
| EC 226-806-4 |
| 4-[4-hydroxyphenyl]butan-2-one |
| Oxyphenylon |
| Himbeerketon |
| Frambione |
| Rheosmine |
| Nat. Raspberry Ketone |
| p-Hydroxy benzylacetone |
| (4-Hydroxybenzyl)acetone |
| 4-hydroxyphenylbutan-2-one |
| SCHEMBL43308 |
| WLN: QR D2V1 |
| P-HYDROXY-BENZYLACETONE |
| RASPBERRY KETONE [MI] |
| NATURAL RASPBERRY KETONE |
| 4-(p-Hydroxyphenyl)2-Butanone |
| RASPBERRY KETONE [INCI] |
| DTXCID3024495 |
| RASPBERRY KETONE [VANDF] |
| FEMA 2588 |
| 2-Butanone,4-Hydroxybenzylacetone |
| RASPBERRY KETONE [USP-RS] |
| 4-(4-hydroxyphenyl) butan-2-one |
| 4-(4-hydroxyphenyl)-butan-2-one |
| 2-butanona, 4-(4-hidroxifenil)- |
| HY-N1426 |
| NSC26515 |
| 1-(4-hydroxy-phenyl)-butan-3-one |
| Tox21_301459 |
| 4-(4-Hydroxy-phenyl)-butan-2-one |
| BBL009822 |
| BDBM50315100 |
| s9480 |
| STK801275 |
| AKOS000120840 |
| 4(P-HYDROXYPHENYL)-2-BUTANONE |
| LS-2828 |
| PS-4612 |
| RASPBERRY KETONE [USP IMPURITY] |
| 4-(4-Hydroxyphenyl)-2-butanone, 99% |
| NCGC00255780-01 |
| AC-24193 |
| SY004032 |
| CAS-5471-51-2 |
| DOBUTAMINE IMPURITY B [EP IMPURITY] |
| 4 - (4 - hydroxyphenyl)butan - 2 - one |
| CS-0016855 |
| FT-0616638 |
| FT-0669951 |
| H0604 |
| EN300-18634 |
| 4-(P-HYDROXYPHENYL)-2-BUTANONE [FCC] |
| 4-(P-HYDROXYPHENYL)-2-BUTANONE [FHFI] |
| D70581 |
| 4-(4-HYDROXYPHENYL)BUTAN-2-ONE [WHO-DD] |
| A830340 |
| AE-473/30684056 |
| Q414484 |
| W-105606 |
| 4-(4-Hydroxyphenyl)-2-butanone, >=98%, FCC, FG |
| 4-(4-Hydroxyphenyl)-2-butanone, analytical standard |
| Z87001611 |
| DOBUTAMINE HYDROCHLORIDE IMPURITY B [EP IMPURITY] |
| 4-(4-Hydroxyphenyl)-2-butanone, natural, >=98%, FCC, FG |
| 4-(4-Hydroxyphenyl)-2-butanone, certified reference material, TraceCERT(R) |
| Raspberry ketone, United States Pharmacopeia (USP) Reference Standard |
| RACTOPAMINE HYDROCHLORIDE SUSPENSION IMPURITY, RASPBERRY KETONE- [USP IMPURITY] |
| BKZ |
|
There are more than 10 synonyms. If you wish to see them all click here.
|