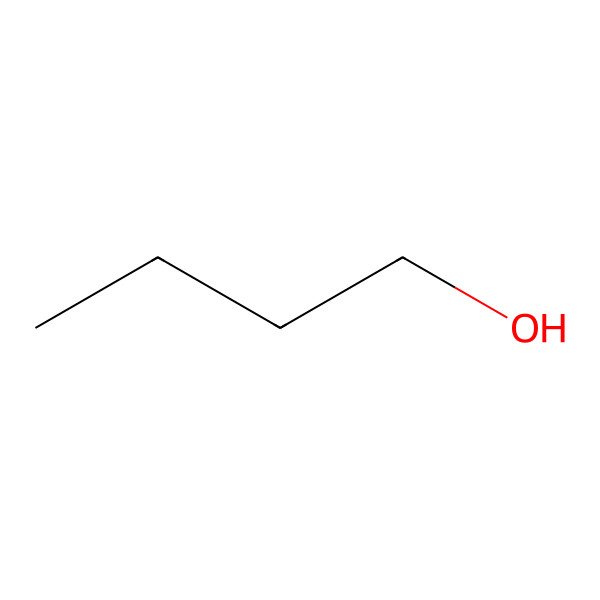
| butanol |
| Butan-1-ol |
| n-butanol |
| Butyl alcohol |
| 71-36-3 |
| n-butyl alcohol |
| 1-hydroxybutane |
| Propylcarbinol |
| Butyl hydroxide |
| Methylolpropane |
| Propylmethanol |
| Hemostyp |
| Butyric alcohol |
| 1-Butyl alcohol |
| n-Butan-1-ol |
| Butanolo |
| Propyl carbinol |
| Alcool butylique |
| Butylowy alkohol |
| BuOH |
| Butanolen |
| Normal primary butyl alcohol |
| Butanols |
| RCRA waste number U031 |
| CCS 203 |
| n-Butylalkohol |
| Alcohol, Butyl |
| Butyl alcohol (natural) |
| FEMA No. 2178 |
| FEMA Number 2178 |
| n-Propyl carbinol |
| 1 Butanol |
| Butanol [French] |
| Butanolen [Dutch] |
| Butyric or normal primary butyl alcohol |
| Butanolo [Italian] |
| n-BuOH |
| 35296-72-1 |
| HSDB 48 |
| NSC 62782 |
| CCRIS 4321 |
| Alcool butylique [French] |
| Butylowy alkohol [Polish] |
| butanol-1 |
| EINECS 200-751-6 |
| UNII-8PJ61P6TS3 |
| Butanol, 1- |
| 8PJ61P6TS3 |
| Butyl alcohol (NF) |
| Butyl alcohol [NF] |
| ALCOHOL,BUTYL |
| DTXSID1021740 |
| CHEBI:28885 |
| AI3-00405 |
| n-Butyl--d6 Alcohol |
| MFCD00002964 |
| NSC-62782 |
| RCRA waste no. U031 |
| UNII-WB09NY83YA |
| n-Butyl-1,1-d2 Alcohol |
| 1-Butanol-4,4,4-d3 |
| CHEMBL14245 |
| DTXCID701740 |
| EC 200-751-6 |
| NCGC00090961-02 |
| Tilcom TNBT |
| Tyzor BP |
| Butyl orthotitanate |
| Tyzor BTM |
| Tyzor TBT |
| 1-BUTANOL-3,3,4,4,4-D5 |
| Orgatix T 25 |
| Orgatix TA 25 |
| Titanium tetrabutoxy- |
| BUTYL ALCOHOL (II) |
| BUTYL ALCOHOL [II] |
| Titanium tetrabutoxide |
| Titanium tetrabutylate |
| Tetra-n-butoxytitanium |
| Tetra-n-butil titanato |
| Tetra-n-butyl titanate |
| Titanium, tetrabutoxy- |
| 32586-14-4 |
| 64118-16-7 |
| Tetrabutoxytitanium(IV) |
| Tetrabutyl orthotitanate |
| 1-Butanol, analytical standard |
| BUTYL ALCOHOL (MART.) |
| BUTYL ALCOHOL [MART.] |
| titanium tetra-n-butoxide |
| 1-BUTANOL-2,2,3,3,4,4,4-D7 |
| Titanium(IV) n-Butoxide |
| TETRABUTYL TITANATE |
| titanato de butilo (IV) |
| 1219794-84-9 |
| C(CC)CO |
| Tetrakis(butanolato)titanium |
| VANADIUM TETRABUTOXIDE |
| TBT-B 1 |
| Tetraortotitanato de n-butilo |
| n Butanol |
| Titanium tetrakis(1-butoxide) |
| n Butyl Alcohol |
| Titanic acid, tetrabutyl ester |
| 1-Butanol, titanium(4) salt |
| C4H10O.1/4Ti |
| Titanium butoxide (Ti(OBu)4) |
| 1-Butanol, ACS reagent, >=99.4% |
| 1-Butanol, titanium(4+) salt |
| CAS-71-36-3 |
| Alcohol, n-Butyl |
| Butyl titanate(IV) (6CI7CI) |
| AKT 850 |
| Butyl alcohol, titanium(4) salt |
| TBT 100 |
| 1-Butanol titanium salt (4:1) |
| 1-Butanol, titanium(4++) salt |
| 1BO |
| Butyl alcohol, titanium(4+) salt |
| Butyl titanate(IV) ((BuO)4Ti) |
| C4-H10-O.1/4Ti |
| 1-butanol, sal de titanio (4 +) |
| 1-Butanol titanium(4+) salt (9CI) |
| Tetrabutyl titanate; (Butyl titanate) |
| Butyl alcohol titanium(4+) salt (8CI) |
| TRIBUTYL ACETYLCITRATE IMPURITY D (EP IMPURITY) |
| TRIBUTYL ACETYLCITRATE IMPURITY D [EP IMPURITY] |
| 1-butanol, sal de titanio (4 +) (4:1) |
| butaneol |
| butylalcohol |
| propilcarbinol |
| butyl-alcohol |
| n-butylalcohol |
| normal butanol |
| 1-butylalcohol |
| n-Propylcarbinol |
| alcohol n-butilo |
| n-Butanolbutanolen |
| nBuOH |
| 1 -butanol |
| 1- butanol |
| 1-n-Butanol |
| Butyl alcohol, n- |
| 1-Butanol, anhydrous |
| Butan- 1- ol |
| Butyl alcohol (8CI) |
| butan - 1 - ol |
| N-Butyl Alcohol,(S) |
| BAN (CHRIS Code) |
| 1-Butanol, for HPLC |
| n-Butanol, HPLC grade |
| 1-Butanol, 99% |
| 6167-45-9 |
| B 1 |
| 1-Butanol, HPLC Grade |
| n-C4H9OH |
| bmse000447 |
| 1-butanol (butyl alcohol) |
| 1-Butanol, 99.9% |
| BUTYL ALCOHOL [FCC] |
| WB09NY83YA |
| BUTYL ALCOHOL [FHFI] |
| BUTYL ALCOHOL [HSDB] |
| WLN: Q4 |
| butan-1-olate,vanadium(4+) |
| 1-BUTANOL [USP-RS] |
| ALCOHOL,BUTYL [VANDF] |
| BIDD:ER0611 |
| N-BUTYL ALCOHOL [MI] |
| Nat. Butanol (Butyl alcohol) |
| Aqualine™ Standard 5.1 |
| N-BUTYL ALCOHOL [INCI] |
| 1-Butanol, LR, >=99% |
| Aqualine™ Standard 10.1 |
| BDBM36173 |
| Butyl Alcohol (Fragrance Grade) |
| Butyl Alcohol Reagent Grade ACS |
| 1-Butanol, anhydrous, 99.8% |
| Butyl Alcohol (Industrial Grade) |
| N-Butanol, ACS, 99.4+% |
| N-BUTYL ALCOHOL [WHO-DD] |
| 1-Butanol, AR, >=99.5% |
| 1-Butanol, for HPLC, 99.8% |
| Butyl alcohol, >=99.9%, FCC |
| NSC62782 |
| Tox21_111046 |
| Tox21_200741 |
| LMFA05000109 |
| STL264186 |
| AKOS000249218 |
| 1-Butanol 500 microg/mL in Methanol |
| 1-Butanol, for HPLC, >=99.7% |
| DB02145 |
| LS-1603 |
| Butyl alcohol, >=99.9%, FCC, FG |
| NCGC00090961-01 |
| NCGC00090961-03 |
| NCGC00258295-01 |
| BP-30034 |
| 1-Butanol, SAJ first grade, >=99.0% |
| 1-Butanol, for molecular biology, >=99% |
| 1-Butanol, JIS special grade, >=99.0% |
| 1-Butanol, p.a., ACS reagent, 99.4% |
| 1-Butanol, for HPLC, >=99.8% (GC) |
| 1-Butanol, spectrophotometric grade, 99.5% |
| 1-Butanol, UV HPLC spectroscopic, 99.5% |
| B0228 |
| B0704 |
| B0944 |
| FT-0607555 |
| FT-0623296 |
| FT-0774976 |
| 1-Butanol, anhydrous, ZerO2(TM), 99.8% |
| EN300-19305 |
| 1-Butanol, Ultrapure, Spectrophotometric Grade |
| Butyl alcohol, natural, >=99.5%, FCC, FG |
| C06142 |
| D03200 |
| Q16391 |
| F0001-1830 |
| InChI=1/C4H10O/c1-2-3-4-5/h5H,2-4H2,1H |
| 1-Butanol, puriss. p.a., ACS reagent, >=99.5% (GC) |
| BDBC6468-886D-4F6C-8746-734F2B63E6CE |
| 1-Butanol, ACS reagent, reag. ISO, reag. Ph. Eur., 99.5% |
| 1-Butanol, United States Pharmacopeia (USP) Reference Standard |
| 1-Butanol, Pharmaceutical Secondary Standard; Certified Reference Material |
| 1-Butanol, puriss. p.a., ACS reagent, reag. ISO, reag. Ph. Eur., >=99.5% (GC) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|