Spermidine
| Internal ID | f7688dfa-9a38-4283-a2d1-25d74ba0b88e |
| Taxonomy | Organic nitrogen compounds > Organonitrogen compounds > Amines > Secondary amines > Dialkylamines |
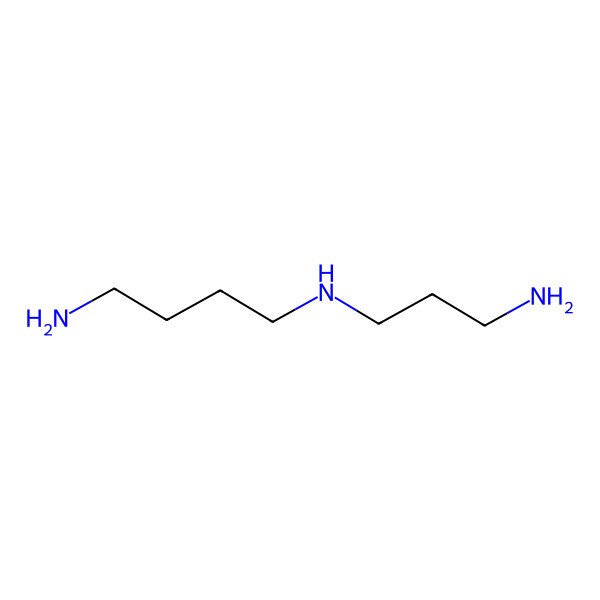
| IUPAC Name | N'-(3-aminopropyl)butane-1,4-diamine |
| SMILES (Canonical) | C(CCNCCCN)CN |
| SMILES (Isomeric) | C(CCNCCCN)CN |
| InChI | InChI=1S/C7H19N3/c8-4-1-2-6-10-7-3-5-9/h10H,1-9H2 |
| InChI Key | ATHGHQPFGPMSJY-UHFFFAOYSA-N |
| Popularity | 18,561 references in papers |
| Molecular Formula | C7H19N3 |
| Molecular Weight | 145.25 g/mol |
| Exact Mass | 145.157897619 g/mol |
| Topological Polar Surface Area (TPSA) | 64.10 Ų |
| XlogP | -1.00 |
| 124-20-9 |
| 1,5,10-Triazadecane |
| 4-Azaoctamethylenediamine |
| N1-(3-Aminopropyl)butane-1,4-diamine |
| Spermidin |
| 4-Azaoctane-1,8-diamine |
| N-(3-aminopropyl)butane-1,4-diamine |
| 1,4-Butanediamine, N-(3-aminopropyl)- |
| N-(3-Aminopropyl)-1,4-butanediamine |
| 1,8-Diamino-4-azaoctane |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL2903 | P16050 | Arachidonate 15-lipoxygenase |
12589.3 nM |
Potency |
via CMAUP
|
| CHEMBL261 | P00915 | Carbonic anhydrase I |
1400 nM |
Ki |
PMID: 20590092
|
| CHEMBL205 | P00918 | Carbonic anhydrase II |
1110 nM |
Ki |
PMID: 20590092
|
| CHEMBL2885 | P07451 | Carbonic anhydrase III |
11500 nM |
Ki |
PMID: 20590092
|
| CHEMBL3729 | P22748 | Carbonic anhydrase IV |
112 nM 112 nM |
Ki Ki |
PMID: 20590092
via Super-PRED |
| CHEMBL3594 | Q16790 | Carbonic anhydrase IX |
1370 nM |
Ki |
PMID: 20590092
|
| CHEMBL4789 | P35218 | Carbonic anhydrase VA |
1220 nM |
Ki |
PMID: 20590092
|
| CHEMBL3969 | Q9Y2D0 | Carbonic anhydrase VB |
1440 nM |
Ki |
PMID: 20590092
|
| CHEMBL3025 | P23280 | Carbonic anhydrase VI |
1410 nM |
Ki |
PMID: 20590092
|
| CHEMBL2326 | P43166 | Carbonic anhydrase VII |
1230 nM |
Ki |
PMID: 20590092
|
| CHEMBL3242 | O43570 | Carbonic anhydrase XII |
44100 nM |
Ki |
PMID: 20590092
|
| CHEMBL3510 | Q9ULX7 | Carbonic anhydrase XIV |
1000 nM |
Ki |
PMID: 20590092
|
| CHEMBL3356 | P05177 | Cytochrome P450 1A2 |
31622.78 nM |
AC50 |
via CMAUP
|
| CHEMBL3397 | P11712 | Cytochrome P450 2C9 |
12589.25 nM |
AC50 |
via CMAUP
|
| CHEMBL2842 | P42345 | Serine/threonine-protein kinase mTOR |
23280.9 nM 26121.6 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL1293256 | P40225 | Thrombopoietin |
6309.6 nM 6309.6 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL1963 | P16473 | Thyroid stimulating hormone receptor |
31622.8 nM |
Potency |
via CMAUP
|
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL3492 | P49721 | Proteasome Macropain subunit | 90.65% | 90.24% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 89.05% | 96.09% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 87.90% | 91.11% |
| CHEMBL1907594 | P30926 | Neuronal acetylcholine receptor; alpha3/beta4 | 87.28% | 97.23% |
| CHEMBL3038469 | P24941 | CDK2/Cyclin A | 84.97% | 91.38% |
| CHEMBL2916 | O14746 | Telomerase reverse transcriptase | 84.29% | 90.00% |
| CHEMBL3230 | O95977 | Sphingosine 1-phosphate receptor Edg-6 | 83.82% | 94.01% |
| CHEMBL4581 | P52732 | Kinesin-like protein 1 | 83.29% | 93.18% |
| CHEMBL3060 | Q9Y345 | Glycine transporter 2 | 80.13% | 99.17% |
| CHEMBL3892 | Q99500 | Sphingosine 1-phosphate receptor Edg-3 | 80.13% | 97.29% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| PubChem | 1102 |
| NPASS | NPC193536 |
| ChEMBL | CHEMBL19612 |
| LOTUS | LTS0061428 |
| wikiData | Q418834 |