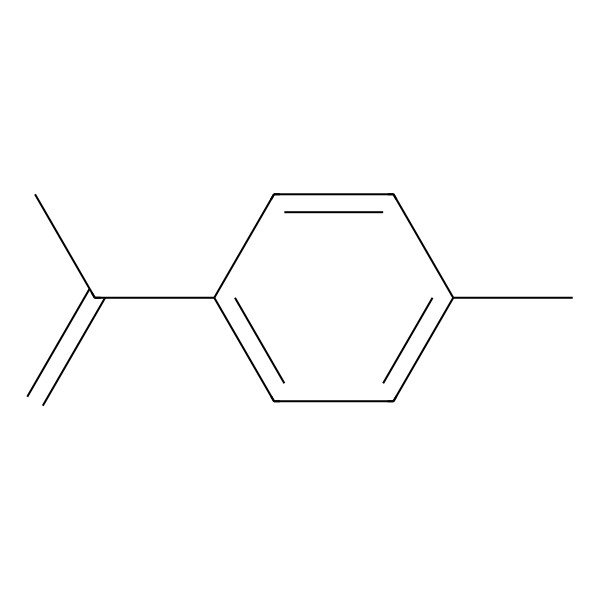
| 1-Methyl-4-(prop-1-en-2-yl)benzene |
| 4-Isopropenyltoluene |
| alpha,p-Dimethylstyrene |
| Dehydro-p-cymene |
| 2-P-Tolylpropene |
| 1-Methyl-4-(1-methylethenyl)benzene |
| 1-Methyl-4-isopropenylbenzene |
| 4-Methylisopropenylbenzene |
| 2-(P-Methylphenyl)propene |
| p-cymenene |
| Isopropenyltoluene, p- |
| p,alpha-Dimethylstyrene |
| p-alpha-Dimethylstyrene |
| 1-Isopropenyl-4-methylbenzene |
| 1-methyl-4-prop-1-en-2-ylbenzene |
| p-alpha-Dimethyl styrene |
| Methyl-p-isopropenylbenzene |
| alpha-Methyl-P-methylstyrene |
| 4-Methyl-alpha-methylstyrene |
| BENZENE, 1-METHYL-4-(1-METHYLETHENYL)- |
| FEMA No. 3144 |
| p-Mentha-1,3,5,8-tetraene |
| Styrene, P-alpha-dimethyl- |
| NSC 361058 |
| Styrene, p,.alpha.-dimethyl- |
| p-Isopropenyl toluene |
| EINECS 214-795-9 |
| Dimethylstyrene, p-alpha- |
| p,.alpha.-Dimethylstyrene |
| 1-methyl-4-prop-1-en-2-yl-benzene |
| UNII-XC8HY3OB86 |
| XC8HY3OB86 |
| ghl.PD_Mitscher_leg0.317 |
| Methyl-4-(1-methylethenyl)-benzene |
| NSC-361058 |
| 1-Methyl-4-(1-methylethenyl)-benzene |
| p-Methyl-alpha-methylstyrene |
| cymenene |
| p-isopropenyltoluene |
| NSC361058 |
| Isopropenyl toluene c |
| p,a - dimethylstyrene |
| P,alpha-Dimethylstyrol |
| alpha-p dimethylstyrene |
| 4,alpha-Dimethylstyrene |
| alpha,4-Dimethylstyrene |
| alpha-Dimethyl-p-styrene |
| p,alpha-Dimethyl-Styrene |
| p-alpha-Dimethyl-Styrene |
| .alpha.,p-Dimethylstyrene |
| p,a-Dimethylstyrene, 8CI |
| .alpha.,4-Dimethylstyrene |
| para-alpha-dimethyl styrene |
| Para- alpha-dimethylstyrene |
| 2-(4-Methylphenyl)propene |
| 1-p-Tolyl-1-methylethylene |
| 1-Methyl-4alpha-methylstyrene |
| para- .alpha.-Dimethylstyrene |
| p-Methyl-.alpha.-methylstyrene |
| 4-Methyl-.alpha.-methylstyrene |
| DTXSID7047564 |
| 1-methyl-4.alpha.-methylstyrene |
| CHEBI:89920 |
| FEMA 3144 |
| 1-Isopropenyl-4-methylbenzene # |
| 4-CH3C6H4C(CH3)CH2 |
| P-.ALPHA.-DIMETHYL STYRENE |
| BAA19532 |
| DIMETHYLSTYRENE, P-.ALPHA.- |
| MFCD00036510 |
| AKOS012525086 |
| STYRENE, P-.ALPHA.-DIMETHYL- |
| BENZENE, 1-METHYL-4-ISOPROPENYL- |
| P-.ALPHA.-DIMETHYL STYRENE [FHFI] |
| 1-Methyl-4-(1-methylethenyl)benzene, 9CI |
| LS-178773 |
| FT-0622103 |
| I0693 |
| p,alpha-Dimethylstyrene, >=98%, stabilized |
| F17145 |
| A804307 |
| Q27162104 |
| alpha,4-Dimethylstyrene, stabilized with 4-t-butylcatechol |
| InChI=1/C10H12/c1-8(2)10-6-4-9(3)5-7-10/h4-7H,1H2,2-3H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|