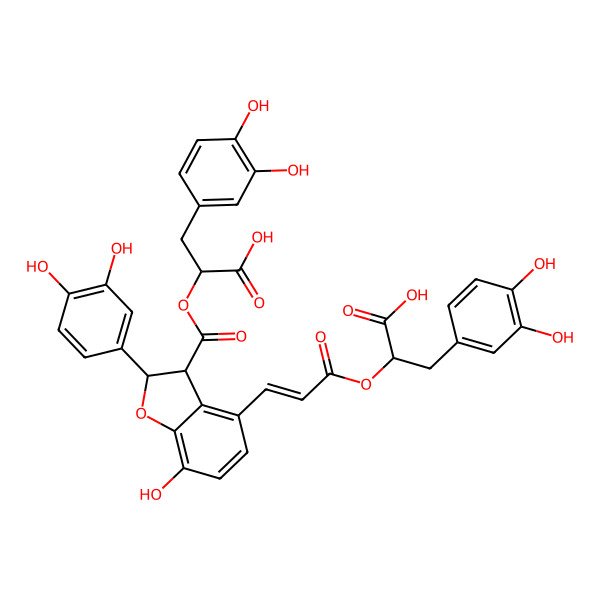
| Lithospermic acid B |
| Dan Shen Suan B |
| 121521-90-2 |
| Danfensuan B |
| Monardic acid B |
| UNII-C1GQ844199 |
| C1GQ844199 |
| CHEBI:134301 |
| ZINC-49538628 |
| 115939-25-8 |
| (2R)-2-[(E)-3-[(2S,3S)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]carbonyl-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-4-yl]prop-2-enoyl]oxy-3-(3,4-dihydroxyphenyl)propanoic acid |
| (2S,3S)-4-((1E)-3-((1R)-1-CARBOXY-2-(3,4-DIHYDROXYPHENYL)ETHOXY)-3-OXO-1-PROPEN-1-YL)-2-(3,4-DIHYDROXYPHENYL)-2,3-DIHYDRO-7-HYDROXY-3-BENZOFURANCARBOXYLIC ACID 3-((1R)-1-CARBOXY-2-(3,4-DIHYDROXYPHENYL)ETHYL) ESTER |
| 3-(1-Carboxy-2-(3,4-dihydroxyphenyl)ethyl) 4-(3-(1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxo-1-propenyl)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-3-benzofurancarboxylate (2S-(2alpha,3beta(S*),4(E(S*))))- |
| SALVIANOLIC ACID B (USP-RS) |
| SALVIANOLIC ACID B [USP-RS] |
| (2R)-2-({(2E)-3-[(2S,3S)-3-{[(1R)-1-Carboxy-2-(3,4-dihydroxyphenyl)ethoxy]carbonyl}-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-4-yl]prop-2-enoyl}oxy)-3-(3,4-dihydroxyphenyl)propanoic acid |
| (R)-2-(((2S,3S)-4-((E)-3-((R)-1-Carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxoprop-1-en-1-yl)-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydrobenzofuran-3-carbonyl)oxy)-3-(3,4-dihydroxyphenyl)propanoic acid |
| 3-Benzofurancarboxylic acid, 4-(3-(1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxo-1-propenyl)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-, 3-(1-carboxy-2-(3,4-dihydroxyphenyl)ethyl) ester, (2S-(2alpha,3beta(S*),4(E(S*))))- |
| 3-Benzofurancarboxylic acid,4-[(1E)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]-3-oxo-1-propenyl]-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-,3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl] ester, (2R,3R)- |
| C36H30O16 |
| C36-H30-O16 |
| (2R)-2-(((2E)-3-((2S,3S)-3-(((1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy)carbonyl)-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-4-yl)prop-2-enoyl)oxy)-3-(3,4-dihydroxyphenyl)propanoic acid |
| (2R)-2-{[(2E)-3-[(2S,3S)-3-{[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]carbonyl}-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-4-yl]prop-2-enoyl]oxy}-3-(3,4-dihydroxyphenyl)propanoic acid |
| (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-[(2S,3S)-2-(3,4-dihydroxyphenyl)-3-[(1R)-1-[(3,4-dihydroxyphenyl)methyl]-2-hydroxy-2-oxo-ethoxy]carbonyl-7-hydroxy-2,3-dihydrobenzofuran-4-yl]prop-2-enoyl]oxy-propanoic acid |
| CHEMBL1615434 |
| SCHEMBL19512041 |
| SALVIANOLIC ACID B [INCI] |
| DTXSID201031347 |
| (2R-(2alpha,3beta(R*),4(E(R*))))-3-(1-Carboxy-2-(3,4-dihydroxyphenyl)ethyl) 4-(3-(1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxo-1-propenyl)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-3-benzofurancarboxylate |
| HY-N1362 |
| SALVIANOLIC ACID B [WHO-DD] |
| AKOS037514825 |
| CCG-270402 |
| CS-5615 |
| Salvianolic acid B, >=94% (HPLC) |
| Salvianolic acid B, analytical standard |
| S4735 |
| Q-100077 |
| Q27275057 |
| SALVIANOLIC ACID B (CONSTITUENT OF CHINESE SALVIA) |
| SALVIANOLIC ACID B (CONSTITUENT OF CHINESE SALVIA) [DSC] |
| Salvianolic acid B, European Pharmacopoeia (EP) Reference Standard |
| Salvianolic acid B, United States Pharmacopeia (USP) Reference Standard |
| (R)-2-(((2S,3S)-4-((E)-3-((R)-1-Carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxoprop-1-en-1-yl)-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydrobenzofuran-3-carbonyl)oxy)-3-(3,4-dihydroxyphenyl)propanoicacid |
| 3-Benzofurancarboxylic acid, 4-[(1E)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]-3-oxo-1-propen-1-yl]-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-, 3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl] ester, (2S,3S)- |
| 4-{(E)-2-[1-Carboxy-2-(3,4-dihydroxy-phenyl)-ethoxycarbonyl]-vinyl}-2-(3,4-dihydroxy-phenyl)-7-hydroxy-2,3-dihydro-benzofuran-3-carboxylic acid 2-carboxy-3-(3,4-dihydroxy-phenyl)-propyl ester |
|
There are more than 10 synonyms. If you wish to see them all click here.
|