| 65-23-6 |
| vitamin B6 |
| Pyridoxol |
| Pyridoxin |
| Gravidox |
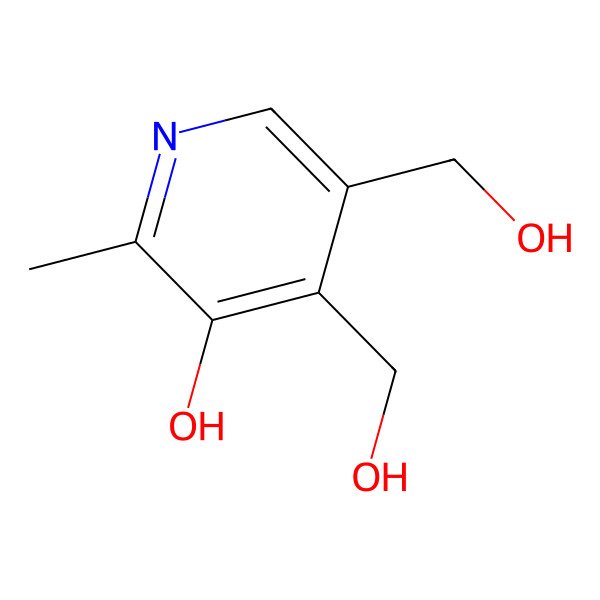
| 3-hydroxy-4,5-bis(hydroxymethyl)-2-methylpyridine |
| Adermine |
| Hydoxin |
| Pyridoxolum |
| 3,4-Pyridinedimethanol, 5-hydroxy-6-methyl- |
| 4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol |
| 5-Hydroxy-6-methyl-3,4-pyridinedimethanol |
| Pyridoxinum |
| Piridossina |
| Piridoxina |
| Piridossina [DCIT] |
| 3-Hydroxy-4,5-dimethylol-alpha-picoline |
| Pyridoxinum [INN-Latin] |
| Piridoxina [INN-Spanish] |
| Hexabione |
| 12001-77-3 |
| 2-Picoline-4,5-dimethanol, 3-hydroxy- |
| Pyridoxine [INN:BAN] |
| (5-Hydroxy-6-methylpyridine-3,4-diyl)dimethanol |
| 2-Methyl-3-hydroxy-4,5-di(hydroxymethyl)pyridine |
| Vitamin V6 |
| 2-Methyl-3-hydroxy-4,5-bis(hydroxymethyl)pyridine |
| 2-Methyl-4,5-bis(hydroxymethyl)-3-hydroxypyridine |
| CHEBI:16709 |
| Vitaped |
| EINECS 200-603-0 |
| Adermin |
| Bezatin |
| Pirivitol |
| 4,5-bis(hydroxymethyl)-2-methyl-pyridin-3-ol |
| UNII-KV2JZ1BI6Z |
| KV2JZ1BI6Z |
| VitaminB6 |
| Infuvite Pediatric |
| Pyridoxin hydrochloride |
| vitamin B-6 |
| 2-methyl-3-hydroxy-4,5-dihydroxymethylpyridine |
| NSC-759148 |
| DTXSID4023541 |
| Pyridoxine free base |
| Pyridoxine (Vit B6) |
| 2-Methyl-3-hydroxy-4,5-dihydroxymethyl-pyridin [German] |
| 4,5-Bis(hydroxymethyl)-2-methyl-3-pyridinol |
| Cernevit-12 |
| Prestwick0_000623 |
| Prestwick1_000623 |
| Prestwick2_000623 |
| Prestwick3_000623 |
| 2-Methyl-3-hydroxy-4,5-dihydroxymethyl-pyridin |
| M.V.I.-12 Lyophilized |
| TimTec1_000657 |
| Oprea1_061614 |
| BSPBio_000586 |
| CBDivE_015627 |
| SPBio_002805 |
| DTXCID903541 |
| BPBio1_000646 |
| Pyridoxine (INN) |
| M.V.I.-12 |
| STK177324 |
| 65-23-6 (FREE BASE) |
| 2-methyl-3-hydroxy-4,5-bis(hydroxy-methyl) pyridine |
| 3-hydroxy-2-Picoline-4,5-dimethanol |
| NSC 759148 |
| DB00165 |
| CAS-58-56-0 |
| SMP2_000230 |
| NCGC00016261-01 |
| NCGC00016261-03 |
| LS-134393 |
| PYRIDOXINE [INN] |
| C00314 |
| AC-907/25014218 |
| PN |
| Vitamin B6, hydrochloride |
| CAS-65-23-6 |
| AIDS006784 |
| AIDS-006784 |
| NSC36225 |
| 2-methyl-4,5-dimethylol-pyridin-3-ol |
| SR-05000001644 |
| NCGC00164317-01 |
| 58-56-0 (HCL) |
| pyridoxina |
| 4,5-bis(hydroxymethyl)-2-methylpyridine-3-ol |
| Pridoxine |
| vitaminum b6 |
| 5-[Dideuterio(hydroxy)methyl]-4-(hydroxymethyl)-2-methylpyridin-3-ol |
| Beesix (Salt/Mix) |
| nchembio.93-comp1 |
| TRIDMAC |
| Becilan (Salt/Mix) |
| Benadon (Salt/Mix) |
| Hexobion (Salt/Mix) |
| Vitamin B6 (TN) |
| Pyridoxol; Vitamin B6 |
| Pyridoxine, >=98% |
| Hexabetalin (Salt/Mix) |
| PYRIDOXINE [MI] |
| Vitamin b6,hydrochloride |
| PYRIDOXINE [INCI] |
| PYRIDOXINE [VANDF] |
| bmse000288 |
| D0X8HP |
| SCHEMBL3506 |
| CHEMBL1364 |
| PYRIDOXINE [WHO-DD] |
| BIDD:PXR0180 |
| VITAMIN B6 [VANDF] |
| P5669_SIGMA |
| PYRIDOXINE [ORANGE BOOK] |
| A11HA02 |
| NSC36225 (HCL) |
| Pyridoxol, Vitamin B6, Gravidox |
| HMS2093L07 |
| KUC106691N |
| Pharmakon1600-01505453 |
| BCP27975 |
| HY-B1328 |
| Tox21_113644 |
| Tox21_300365 |
| BBL005552 |
| BDBM50103505 |
| c1302 |
| MFCD00006335 |
| NSC759148 |
| s3980 |
| ZINC00049154 |
| AKOS005410791 |
| Tox21_113644_1 |
| CCG-213453 |
| CS-O-02556 |
| CS-W019950 |
| Vitamin B6 100 microg/mL in Methanol |
| NCGC00016261-02 |
| NCGC00016261-04 |
| NCGC00016261-05 |
| NCGC00016261-08 |
| NCGC00164317-02 |
| NCGC00254340-01 |
| AC-14512 |
| DS-11013 |
| KSC-11-207-23 |
| SBI-0206844.P001 |
| 3,4-piridindimetanol, 5-hidroxi-6-metil- |
| 3-Hydroxy-4,5-dimethylol-.alpha.-picoline |
| AM20070169 |
| FT-0631288 |
| FT-0674200 |
| EN300-39851 |
| D08454 |
| O10129 |
| 4,5-Bis(hydroxymethyl)-2-methyl-3-pyridinol # |
| A835033 |
| Q423746 |
| Q-201646 |
| SR-05000001644-1 |
| SR-05000001644-3 |
| 2-Methyl-3-oxylato-4,5-bis(hydroxymethyl)pyridinium |
| 2B3E07D2-E4CC-4CC5-B085-6070BA01F9F0 |
| Z382721012 |
| 2-methyl-3-hydroxy-4-hydroxymethyl-5-hydroxymethyl pyridine |
| InChI=1/C8H11NO3/c1-5-8(12)7(4-11)6(3-10)2-9-5/h2,10-12H,3-4H2,1H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|