| 23180-57-6 |
| Peoniflorin |
| Paeonia moutan |
| NSC 178886 |
| UNII-21AIQ4EV64 |
| 21AIQ4EV64 |
| CCRIS 6494 |
| EINECS 245-476-2 |
| PAEONIFLORIN (USP-RS) |
| PAEONIFLORIN [USP-RS] |
| NSC-178886 |
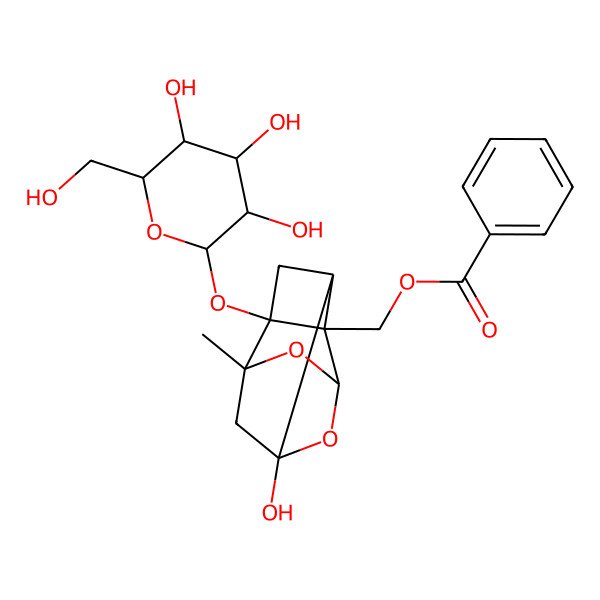
| ((2S,2aR,2a1S,3aR,4R,5aR)-4-Hydroxy-2-methyl-2a-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)hexahydro-2H-1,5-dioxa-2,4-methanocyclobuta[cd]pentalen-2a1-yl)methyl benzoate |
| beta-D-Glucopyranoside, (1aS,2R,3aR,5R,5aR,5bS)-5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta(cd)pentalen-1a(2H)-yl |
| NSC178886 |
| .BETA.-D-GLUCOPYRANOSIDE, (1AR,2S,3AR,5R,5AR,5BS)-5B-((BENZOYLOXY)METHYL)TETRAHYDRO-5-HYDROXY-2-METHYL-2,5-METHANO-1H-3,4-DIOXACYCLOBUTA(CD)PENTALEN-1A(2H)-YL |
| .beta.-D-Glucopyranoside, (1aR,2S,3aR,5R,5aR,5bS)-5b-[(benzoyloxy)methyl]tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta[cd]pentalen-1a(2H)-yl |
| .BETA.-D-GLUCOPYRANOSIDE, 5B-((BENZOYLOXY)METHYL)TETRAHYDRO-5-HYDROXY-2-METHYL-2,5-METHANO-1H-3,4-DIOXACYCLOBUTA(CD)PENTALEN-1A(2H)-YL, (1AR-(1A.ALPHA.,2.BETA.,3A.ALPHA.,5.ALPHA.,5A.ALPHA.,5B.ALPHA.))- |
| .beta.-D-Glucopyranoside, 5b-[(benzoyloxy)methyl]tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta[cd]pentalen-1a(2H)-yl, [1aR-(1a.alpha.,2.beta.,3a.alpha.,5.alpha.,5a.alpha.,5b.alpha.)]- |
| beta-d-Glucopyranoside, 5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta(cd)pentalen-1a(2H)-yl, (1aR-(1aalpha,2beta,3aalpha,5alpha,5aalpha,5balpha))- |
| beta-d-Glucopyranoside, 5b-[(benzoyloxy)methyl]tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta[cd]pentalen-1a(2H)-yl, [1aR-(1aalpha,2beta,3aalpha,5alpha,5aalpha,5balpha)]- |
| PEONIFLORIN [INCI] |
| D0PD0F |
| SCHEMBL549033 |
| CHEBI:7889 |
| CHEMBL4303209 |
| DTXSID2042648 |
| Paeoniflorin, analytical standard |
| Paeoniflorin, >=98% (HPLC) |
| HMS3884D17 |
| 5b-((Benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-lH-3,4-dioxacyclobuta(cd)pentalen-1a(2H)-yl-beta-D-glucopyranoside |
| beta-D-Glucopyranoside, 5b-((benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta(cd)pentalen-1a(2H)-yl, (1aR-(1a-alpha,2-beta,3a-alpha,5-alpha,5a-alpha,5b-alpha))- |
| HY-N0293 |
| s2410 |
| AKOS025311455 |
| CCG-269549 |
| AS-12193 |
| C09959 |
| AB01566855_01 |
| Q-100296 |
| Q7124104 |
| ((2S,2aR,2a1S,3aR,4R,5aR)-4-Hydroxy-2-methyl-2a-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)hexahydro-2H-1,5-dioxa-2,4-methanocyclobuta[cd]pentalen-2a1-yl)methylbenzoate |
| [(1R,2S,3R,5R,6R,8S)-6-hydroxy-8-methyl-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9,10-dioxatetracyclo[4.3.1.02,5.03,8]decan-2-yl]methyl benzoate |
| [hydroxy-methyl-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-[?]yl]methyl benzoate |
| b-D-Glucopyranoside,(1aR,2S,3aR,5R,5aR,5bS)-5b-[(benzoyloxy)methyl]tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H-3,4-dioxacyclobuta[cd]pentalen-1a(2H)-yl |
| beta-D-GLUCOPYRANOSIDE, (1AR,2S,3AR,5R,5AR,5BS)-5B-((BENZOYLOXY)METHYL)TETRAHYDRO-5-HYDROXY-2-METHYL-2,5-METHANO-1H-3,4-DIOXACYCLOBUTA(CD)PENTALEN-1A(2H)-YL |
|
There are more than 10 synonyms. If you wish to see them all click here.
|