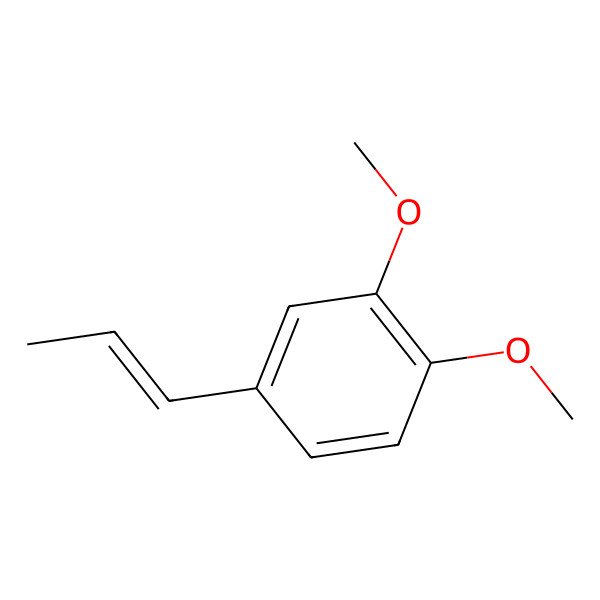
| trans-Methylisoeugenol |
| 93-16-3 |
| Methyl isoeugenol |
| 6379-72-2 |
| Isomethyleugenol |
| 4-trans-Propenylveratrole |
| trans-4-Propenylveratrole |
| Isoeugenol methyl ether |
| trans-isomethyleugenol |
| 4-Propenylveratrole |
| Isohomogenol |
| 1,2-Dimethoxy-4-propenylbenzene |
| Benzene, 1,2-dimethoxy-4-(1-propenyl)- |
| (E)-Methyl isoeugenol |
| Isoeugenyl methyl ether |
| Methylisoeugenol, (E)- |
| 1,2-dimethoxy-4-[(1E)-prop-1-en-1-yl]benzene |
| 1,2-dimethoxy-4-[(E)-prop-1-enyl]benzene |
| (e)-methylisoeugenol |
| 1-Veratryl-1-propene |
| Benzene, 1,2-dimethoxy-4-propenyl-, (E)- |
| Benzene, 1,2-dimethoxy-4-propenyl |
| EINECS 228-958-7 |
| UNII-J6M6C71VVR |
| 4-(1-Propenyl)-1,2-dimethoxybenzene |
| BRN 1911285 |
| 3,4-Dimethoxypropenylbenzene |
| J6M6C71VVR |
| CHEBI:6877 |
| 1,2-dimethoxy-4-(prop-1-en-1-yl)benzene |
| Benzene, 1,2-dimethoxy-4-(1E)-1-propenyl- |
| cis-Methylisoeugenol |
| 4-Prop-1-enylveratrole |
| Veratrole, 4-propenyl- |
| Benzene, 1,2-dimethoxy-4-propenyl- |
| (E)-1,2-Dimethoxy-4-(prop-1-en-1-yl)benzene |
| TRIDEUTEROMETHYLISOEUGENOL |
| 6380-24-1 |
| cis-O-Methylisoeugenol |
| 4-prop-1-enylveratrol |
| 1,2-Dimethoxy-4-[1-propenyl]benzene # |
| 54349-79-0 |
| Benzene, 4-(1-propenyl)-1,2-dimethoxy |
| Benzene, 1,2-dimethoxy-4-(1-propen-1-yl)- |
| DTXSID0052621 |
| 4 - prop - 1 - enylveratrole |
| methylation |
| O-Methyl Isoeugenol |
| ISOHOMOEUGENOL |
| Benceno, 1,2-dimetoxi-4-(1-propen-1-il)- |
| iso eugenol methyl ether |
| Methyl IsoEugenol Natural |
| 1,4-Isoeugenol methyl ether |
| ghl.PD_Mitscher_leg0.366 |
| (E)-O-METHYLISOEUGENOL |
| 1-Propene,4-dimethoxyphenyl)- |
| CHEMBL465829 |
| 4-cis-Propenylveratrole; cis-Isoeugenol methyl ether;cis-Methylisoeugenol |
| DTXCID4031194 |
| WLN: 2U1R CO1 DO1 |
| CHEBI:14469 |
| Benzene,2-dimethoxy-4-propenyl- |
| Methyl isoeugenol, >=98%, FG |
| NNWHUJCUHAELCL-SNAWJCMRSA-N |
| DTXSID501020311 |
| (E)-ISOEUGENOL METHYL ETHER |
| HY-N2439 |
| NSC46111 |
| FEMA NO. 2476, E- |
| Tox21_303830 |
| BBL009809 |
| MFCD00009282 |
| NSC-46111 |
| s3006 |
| STK801268 |
| AKOS005608367 |
| Benzene,2-dimethoxy-4-(1-propenyl)- |
| ISOEUGENYL METHYL ETHER, TRANS- |
| CAS-93-16-3 |
| 1,2-Dimethoxy-4-propenylbenzene, 99% |
| NCGC00357104-01 |
| VERATROLE, 4-PROPENYL-, TRANS- |
| 1,2-dimethoxy-4-prop-1-en-1-ylbenzene |
| AS-56928 |
| LS-29884 |
| VS-02193 |
| 4-(1-Propenyl)pyrocatechol Dimethyl Ether |
| (E)-1,2-dimethoxy-4-(prop-1-enyl)benzene |
| CS-0022657 |
| P1103 |
| (E)-1-(3,4-DIMETHOXYPHENYL)PROPENE |
| EN300-2559873 |
| Benzene, 1,2-dimethoxy-4-(1-propenyl)-, (E)- |
| EN300-21037355 |
| (E)-1,2-DIMETHOXY-4-(1-PROPENYL)BENZENE |
| W-100250 |
| Q10858039 |
| Q27108981 |
| BENZENE,1,2-DIMETHOXY,4-PROPENYL ISOEUGENOL,METHYL ETHER |
|
There are more than 10 synonyms. If you wish to see them all click here.
|