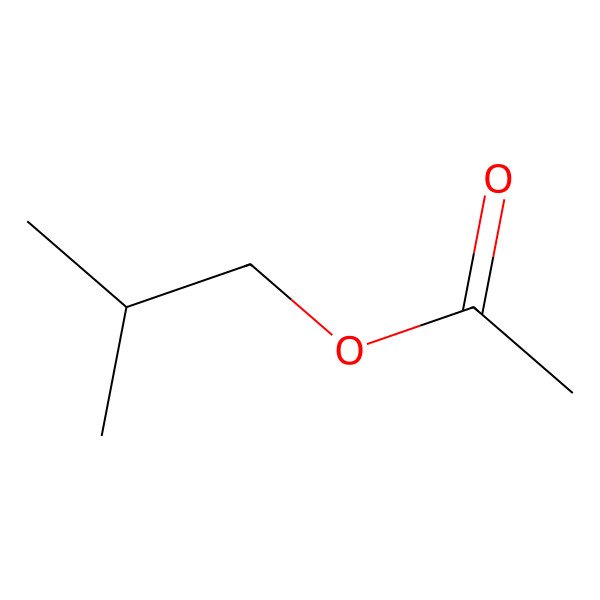
| 110-19-0 |
| 2-Methylpropyl acetate |
| Isobutyl ethanoate |
| Acetic acid, 2-methylpropyl ester |
| 2-Methyl-1-propyl acetate |
| 2-Methylpropyl ethanoate |
| Acetic acid, isobutyl ester |
| Acetate d'isobutyle |
| iso-butyl acetate |
| Isobutylacetat |
| i-butyl acetate |
| beta-Methylpropyl ethanoate |
| Acetic Acid Isobutyl Ester |
| Isobutylazetat |
| Isobutyl acetate (natural) |
| Isobutylester kyseliny octove |
| FEMA No. 2175 |
| FEMA Number 2175 |
| NSC 8035 |
| HSDB 609 |
| UNII-7CR47FO6LF |
| EINECS 203-745-1 |
| 7CR47FO6LF |
| Acetate d'isobutyle [French] |
| BRN 1741909 |
| DTXSID5026837 |
| CHEBI:50569 |
| Essigsaeureisobutylester |
| AI3-15305 |
| NSC-8035 |
| UN1213 |
| Isobutylester kyseliny octove [Czech] |
| .beta.-Methylpropyl ethanoate |
| 2-Methyl-1-propanol, acetate |
| DTXCID906837 |
| EC 203-745-1 |
| 4-02-00-00149 (Beilstein Handbook Reference) |
| Isobutyl acetate, 99% |
| Isobutyl acetate [UN1213] [Flammable liquid] |
| ISOBUTYL ACETATE (USP-RS) |
| ISOBUTYL ACETATE [USP-RS] |
| iso-butylacetate |
| AcOiBu |
| Actate d'isobutyle |
| Isobutyl acetate fcc |
| MFCD00008932 |
| Nat.Isobutyl Acetate |
| Isobutyl acetate, 8CI |
| IBA (CHRIS Code) |
| acetate, 2-Methylpropyl |
| BUTYL-ISO ACETATE |
| Acetic acid-isobutyl ester |
| beta -methylpropyl ethanoate |
| SCHEMBL22678 |
| 2-Methylpropyl acetate, 9CI |
| Isobutyl ester of acetic acid |
| CHEMBL46999 |
| ISOBUTYL ACETATE [MI] |
| Isobutyl Acetate Reagent Grade |
| ISOBUTYL ACETATE [FCC] |
| ISOBUTYL ACETATE [FHFI] |
| ISOBUTYL ACETATE [HSDB] |
| ISOBUTYL ACETATE [INCI] |
| Acetic acid 2-methylpropyl ester |
| FEMA 2175 |
| NSC8035 |
| WLN: 1Y1 & 1OV1 |
| Isobutyl Acetate (Fragrance Grade) |
| 2-Methylpropyl ester of acetic acid |
| Tox21_201735 |
| Isobutyl acetate, analytical standard |
| NA1213 |
| STL280347 |
| AKOS015901357 |
| Isobutyl acetate, >=98%, FCC, FG |
| LS-2847 |
| UN 1213 |
| NCGC00249107-01 |
| NCGC00259284-01 |
| CAS-110-19-0 |
| A0034 |
| FT-0621747 |
| Isobutyl acetate, natural, >=97%, FCC, FG |
| Isobutyl acetate [UN1213] [Flammable liquid] |
| Q420657 |
| J-002396 |
| F0001-0218 |
| Acetic acid-isobutyl ester 1000 microg/mL in Acetonitrile |
| InChI=1/C6H12O2/c1-5(2)4-8-6(3)7/h5H,4H2,1-3H |
| Isobutyl acetate, United States Pharmacopeia (USP) Reference Standard |
|
There are more than 10 synonyms. If you wish to see them all click here.
|