| 14101-61-2 |
| D-gamma-Tocotrienol |
| Plastochromanol |
| 7,8-Dimethyltocotrienol |
| Plastochromanol 3 |
| .gamma.-Tocotrienol |
| D-.gamma.-Tocotrienol |
| (R)-.gamma.-Tocotrienol |
| UNII-185QAE24TR |
| 185QAE24TR |
| Gama-Tocotrienol |
| CHEBI:33277 |
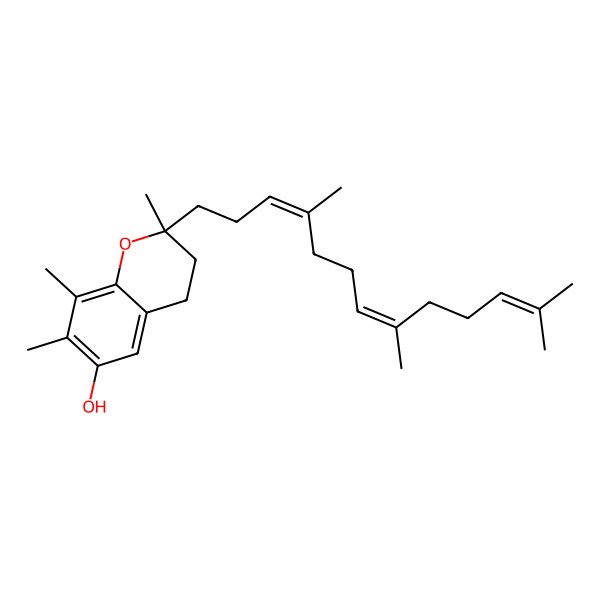
| (2R)-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl]-3,4-dihydrochromen-6-ol |
| (R)-2,7,8-Trimethyl-2-((3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl)chroman-6-ol |
| (R-(E,E))-3,4-Dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-2H-1-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-, (R-(E,E))- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-, [R-(E,E)]- |
| 2H-1-benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-, (2R)- |
| 2R,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl]-3,4-dihydro-2H-chromen-6-ol |
| gammaTocotrienol |
| gamma Tocotrienol |
| gamma -Tocotrienol |
| 2H-1-BENZOPYRAN-6-OL, 3,4-DIHYDRO-2,7,8-TRIMETHYL-2-((3E,7E)-4,8,12-TRIMETHYL-3,7,11-TRIDECATRIEN-1-YL)-, (2R)- |
| 2H-1-BENZOPYRAN-6-OL, 3,4-DIHYDRO-2,7,8-TRIMETHYL-2-((3E,7E)-4,8,12-TRIMETHYL-3,7,11-TRIDECATRIENYL)-, (2R)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrien-1-yl]-, (2R)- |
| Gamma-T3 |
| 2R-gamma-tocotrienol |
| (R)-gamma-Tocotrienol |
| D09YBF |
| BIDD:PXR0016 |
| gammaTocotrienolPlastochromanol |
| CHEMBL120697 |
| SCHEMBL3272929 |
| SCHEMBL16430897 |
| DTXSID101019984 |
| HMS3650C14 |
| GAMMA TOCOTRIENOL [WHO-DD] |
| 3,4-Dihydro-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-2H-1-benzopyran-6-ol |
| Gamma-tocotrienol (prostate cancer) |
| LMPR02020057 |
| gamma-Tocotrienol, analytical standard |
| AKOS040744431 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-tr imethyl-3,7,11-tridecatrienyl]-, (2R)- (9CI) |
| J13.903C |
| HY-108694 |
| CS-0029988 |
| D-gamma-Tocotrienol, analytical reference material |
| SR-01000946268 |
| SR-01000946268-1 |
| Q15633932 |
| Gamma-tocotrienol (prostate cancer), Davos Life Science |
| (R)-2,7,8-trimethyl-2-((3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl)chroman-6-ol |
| 6-Chromanol, 2,7,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)- |
| 6-Chromanol, 2,7,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)- (7CI,8CI) |
| (2R)-2,7,8-trimethyl-2-(4',8',12'-trimethyl-trideca-3',7',11'-trienyl)-6-hydroxychroman |
| (2R)-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl]-3,4-dihydro-2H-chromen-6-ol |
| (r)-2,7,8-trimethyl-2-((3e,7e)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl) chroman-6-ol |
| 153831-49-3 |
| 2,7,8-TRIMETHYL-2-[(3E,7E,11E,15E,19E,23E,27E)-4,8,12,16,20,24,28,32-O CTAMETHYL-3,7,11,15,19,23,27,31-TRITRIACONTAOCTAENYL]-6-CHROMANOL |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-, (2R)- (9CI) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|