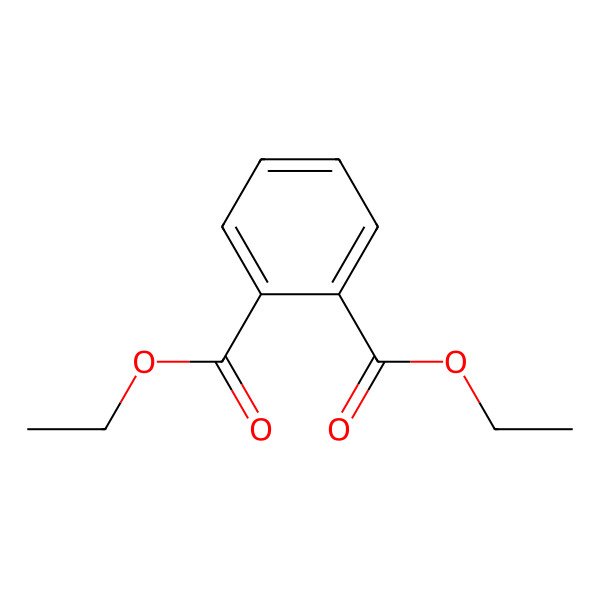
| 84-66-2 |
| Ethyl phthalate |
| phthalic acid diethyl ester |
| Anozol |
| Diethylphthalate |
| diethyl benzene-1,2-dicarboxylate |
| Neantine |
| Phthalol |
| Solvanol |
| Diethyl o-phthalate |
| Palatinol A |
| Placidol E |
| Unimoll DA |
| Phthalsaeurediaethylester |
| o-Bis(ethoxycarbonyl)benzene |
| Diethyl 1,2-benzenedicarboxylate |
| 1,2-Diethyl phthalate |
| Estol 1550 |
| 1,2-Benzenedicarboxylic acid, diethyl ester |
| Di-n-ethyl phthalate |
| o-Benzenedicarboxylic acid diethyl ester |
| Diethyl o-phenylenediacetate |
| Phthalic acid, diethyl ester |
| RCRA waste number U088 |
| Diethylester kyseliny ftalove |
| NCI-C60048 |
| NSC 8905 |
| 1,2-benzenedicarboxylic acid diethyl ester |
| CCRIS 2675 |
| HSDB 926 |
| CHEBI:34698 |
| diethyl phtalate |
| DPX-F5384 |
| NSC-8905 |
| EINECS 201-550-6 |
| UNII-UF064M00AF |
| Diethyl-o-phthalate |
| BRN 1912500 |
| O-phthalic acid, diethyl ester |
| UF064M00AF |
| DTXSID7021780 |
| AI3-00329 |
| Phthalsaeurediaethylester [German] |
| Diethyl phthalate (NF) |
| Diethyl phthalate [NF] |
| Diethylester kyseliny ftalove [Czech] |
| Diethyl Phthalate [USAN] |
| RCRA waste no. U088 |
| 1,2-diethyl benzene-1,2-dicarboxylate |
| DTXCID901780 |
| PHTHALIC ACID ETHYL ESTER |
| EC 201-550-6 |
| 1,2-Benzenedicarboxylic acid, 1,2-diethyl ester |
| 4-09-00-03172 (Beilstein Handbook Reference) |
| NCGC00090974-03 |
| o-Benzenedicarboxylic acid, diethyl ester |
| Diethyl phthalate, 99% |
| DIETHYL PHTHALATE (II) |
| DIETHYL PHTHALATE [II] |
| DIETHYL PHTHALATE (MART.) |
| DIETHYL PHTHALATE [MART.] |
| DIETHYL PHTHALATE (USP-RS) |
| DIETHYL PHTHALATE [USP-RS] |
| CAS-84-66-2 |
| DIETHYL PHTHALATE (EP MONOGRAPH) |
| DIETHYL PHTHALATE [EP MONOGRAPH] |
| 68988-18-1 |
| SMR000857334 |
| diethyl phthalate (dep) |
| Diethyl phthalate, PESTANAL(R), analytical standard |
| Dietylftalat |
| solvano l |
| Kodaflex DEP |
| Diethyl phthalic acid |
| Phthalic acid diethyl |
| diethyl phthalate (o) |
| Diethyl Phthalate, NF |
| DPH (CHRIS Code) |
| DEP (Diethyl phthalate) |
| diethyl 1,2-benzenedioate |
| bmse000846 |
| Epitope ID:140105 |
| WLN: 2OVR BVO2 |
| Diethyl phthalate, >=99% |
| Diethyl phthalate, 99.5% |
| SCHEMBL22296 |
| dimethyphalate ,ethylphthalate |
| MLS001336021 |
| MLS001336022 |
| MLS002152901 |
| MLS002177800 |
| BIDD:ER0639 |
| Diethyl ester of phthalic acid |
| DIETHYL PHTHALATE (0) |
| Phthalic acid, bis-ethyl ester |
| CHEMBL388558 |
| Diethyl phthalate, >=99.5% |
| DIETHYL PHTHALATE [HSDB] |
| DIETHYL PHTHALATE [INCI] |
| Diethyl-1,2-benzenedicarboxylate |
| DIETHYL PHTHALATE [VANDF] |
| ETHYL PHTHALATE [WHO-DD] |
| NSC8905 |
| Diethyl phthalate, LR, >=99% |
| HMS2233J05 |
| HMS3369G01 |
| PHTHALIC ACID,DIETHYL ESTER |
| Diethyl phthalate/dimethyl phthalate |
| STR04116 |
| EINECS 273-520-0 |
| Tox21_111050 |
| Tox21_201874 |
| Tox21_300183 |
| BBL011577 |
| Diethyl phthalate; (Ethyl phthalate) |
| MFCD00009111 |
| STL163320 |
| AKOS000119867 |
| Benzenedicarboxylic acid, diethyl ester |
| LS-1838 |
| PHTHALIC ACID ETHYL ESTER [MI] |
| Diethyl Phthalate MIL-D-242 Mil Spec |
| NCGC00090974-01 |
| NCGC00090974-02 |
| NCGC00090974-04 |
| NCGC00090974-05 |
| NCGC00090974-06 |
| NCGC00254098-01 |
| NCGC00259423-01 |
| Diethyl Phthalate Metal Plastic IBC/Tote |
| benzene-1,2-dicarboxylic acid diethyl ester |
| CS-0013981 |
| FT-0624802 |
| FT-0666787 |
| P0296 |
| Diethyl ester of 1,2-Benzenedicarboxylic acid |
| Diethylphthalate, A-A-59314, JAN-D-242 |
| EN300-20094 |
| D03804 |
| Diethyl phthalate, SAJ special grade, >=98.0% |
| Q419811 |
| Q-200982 |
| F1908-0104 |
| Diethyl phthalate, European Pharmacopoeia (EP) Reference Standard |
| Diethyl phthalate, United States Pharmacopeia (USP) Reference Standard |
| Diethyl Phthalate, Pharmaceutical Secondary Standard; Certified Reference Material |
| InChI=1/C12H14O4/c1-3-15-11(13)9-7-5-6-8-10(9)12(14)16-4-2/h5-8H,3-4H2,1-2H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|