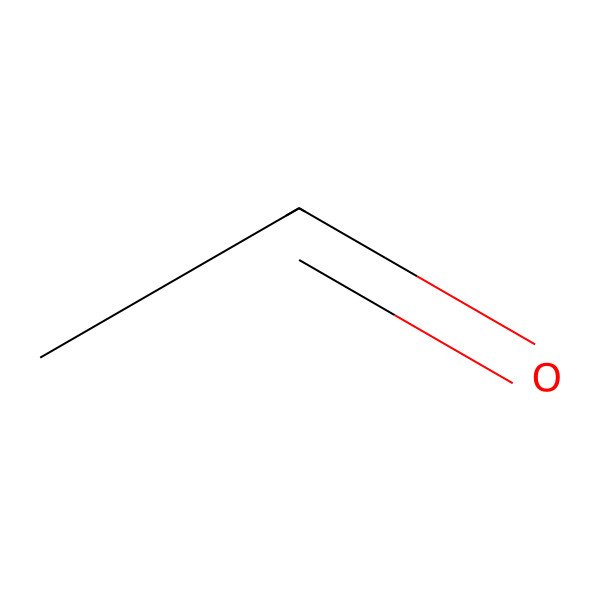
| ethanal |
| 75-07-0 |
| acetic aldehyde |
| ethyl aldehyde |
| Acetaldehyd |
| Acetylaldehyde |
| aldehyde |
| Aldeide acetica |
| Acetic ethanol |
| Octowy aldehyd |
| Aldehyde acetique |
| Ethylaldehyde |
| Azetaldehyd |
| RCRA waste number U001 |
| Acetaldehyde (natural) |
| NSC 7594 |
| acetaldehydes |
| NCI-C56326 |
| FEMA No. 2003 |
| CCRIS 1396 |
| HSDB 230 |
| Acetaldehyd [German] |
| ACETYL GROUP |
| ethaldehyde |
| Acetaldehido |
| CHEBI:15343 |
| Octowy aldehyd [Polish] |
| UNII-GO1N1ZPR3B |
| Aldeide acetica [Italian] |
| GO1N1ZPR3B |
| AI3-31167 |
| Aldehyde acetique [French] |
| EINECS 200-836-8 |
| MFCD00006991 |
| UN1089 |
| DTXSID5039224 |
| CH3CHO |
| NSC-7594 |
| DTXCID202 |
| RCRA waste no. U001 |
| NSC7594 |
| EC 200-836-8 |
| ACETALDEHYDE (IARC) |
| ACETALDEHYDE [IARC] |
| VINYL ALCOHOL (FROM ALCOHOLYSIS OR HYDROLYSIS OF VINYL ACETATE UNITS) |
| Acetaldehyde, >=99%, meets FCC analytical specification |
| acetaldhyde |
| acetoaldehyde |
| Acetaldeyde |
| Aceteldehyde |
| Acetehyde |
| acetic hydride |
| ethan-1-one |
| Acetaldehyde 10% |
| MeCHO |
| Acetaldehyde Natural |
| Aldehyde C(2) |
| ACETALD |
| CH2CHO |
| AAD (CHRIS Code) |
| NATURAL ALDEFRESH |
| ACETALDEHYDE [MI] |
| ACETALDEHYDE [FCC] |
| bmse000647 |
| D0C7WN |
| Epitope ID:145667 |
| ACETALDEHYDE [FHFI] |
| ACETALDEHYDE [HPUS] |
| ACETALDEHYDE [HSDB] |
| ACETALDEHYDE [INCI] |
| WLN: VH1 |
| Pesticide Code: 202300 |
| ACETALDEHYDE [USP-RS] |
| ACETALDEHYDE [WHO-DD] |
| Acetaldehyde, >=99%, FG |
| BIDD:ER0621 |
| Acetaldehyde, >=99%, FCC |
| CHEMBL170365 |
| GTPL6277 |
| Acetaldehyde, analytical standard |
| CHEBI:16571 |
| Program mandated by AB1807.) |
| STR01382 |
| Tox21_202479 |
| Acetaldehyde, natural, >=99%, FG |
| Acetaldehyde, ReagentPlus(R), 99% |
| NA1089 |
| STL264249 |
| AKOS000120180 |
| LS-1654 |
| UN 1089 |
| Acetaldehyde, ACS reagent, >=99.5% |
| CAS-75-07-0 |
| Acetaldehyde, >=99%, FCC, stabilized |
| NCGC00091753-01 |
| NCGC00260028-01 |
| InChI=1/C2H4O/c1-2-3/h2H,1H |
| Acetaldehyde [UN1089] [Flammable liquid] |
| Acetaldehyde, >=90.0%, SAJ first grade |
| Acetaldehyde [UN1089] [Flammable liquid] |
| FT-0621719 |
| FT-0660962 |
| EN300-19152 |
| C00084 |
| D78540 |
| Q61457 |
| Acetaldehyde, ReagentPlus(R), >=99.0% (GC) |
| A838317 |
| BRD-K77914232-001-01-3 |
| Q57695648 |
| Acetaldehyde, puriss. p.a., anhydrous, >=99.5% (GC) |
| F2190-0651 |
| Flavor and Extract Manufacturers' Association Number 2003 |
| Acetaldehyde, United States Pharmacopeia (USP) Reference Standard |
| Acetaldehyde* (These peer-reviewed values were developed under the Toxic Air Contaminant (TAC) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|