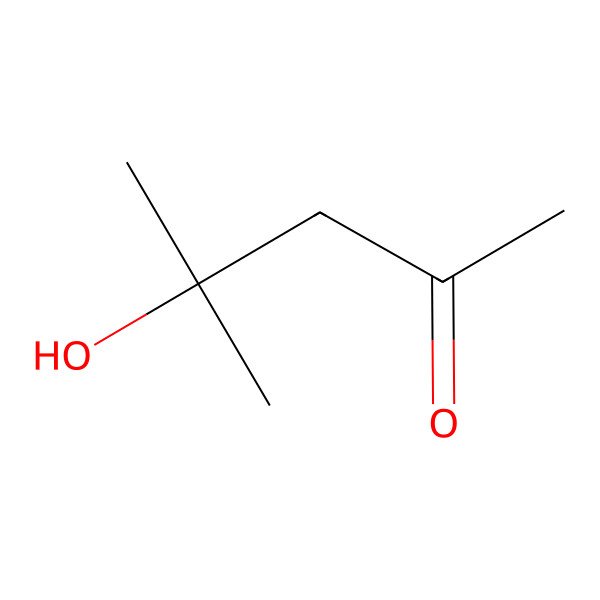
| 4-HYDROXY-4-METHYL-2-PENTANONE |
| 123-42-2 |
| 4-Hydroxy-4-methylpentan-2-one |
| Diacetonalkohol |
| Diketone alcohol |
| Diacetonalcohol |
| Diacetone |
| Pyranton |
| Tyranton |
| Acetonyldimethylcarbinol |
| Diacetone-alcool |
| Pyranton A |
| Diacetonalcool |
| 2-Methyl-2-pentanol-4-one |
| Diacetonyl alcohol |
| 4-Hydroxy-2-keto-4-methylpentane |
| 2-Pentanone, 4-hydroxy-4-methyl- |
| 4-Idrossi-4-metil-pentan-2-one |
| Dimethyl acetonyl carbinol |
| 4-Hydroxy-4-methylpentanone-2 |
| Caswell No. 280 |
| 2-Hydroxy-2-methyl-4-pentanone |
| 4-Hydroxy-4-methyl-pentan-2-on |
| 4-Hydroxy-4-methyl pentan-2-one |
| NSC 9005 |
| CCRIS 6177 |
| Pyraton |
| Diacetonalcohol [Dutch] |
| Diacetonalcool [Italian] |
| Diacetonalkohol [German] |
| HSDB 1152 |
| Diacetone-alcool [French] |
| UNII-Q7WP157PTD |
| EINECS 204-626-7 |
| Q7WP157PTD |
| EPA Pesticide Chemical Code 033901 |
| BRN 1740440 |
| DTXSID6024917 |
| CHEBI:55381 |
| AI3-00045 |
| Pentanone, 4-hydroxy-4-methyl- |
| NSC-9005 |
| UN1148 |
| 4-Hydroxy-4-methylpentanone |
| 4-Methyl-2-pentanon-4-ol |
| DTXCID304917 |
| 4-Idrossi-4-metil-pentan-2-one [Italian] |
| 4-Methyl-4-hydroxy-2-pentanone |
| EC 204-626-7 |
| 4-01-00-04023 (Beilstein Handbook Reference) |
| 4-Hydroxy-4-methyl-pentan-2-on [German, Dutch] |
| (CH3)2C(OH)CH2C(O)CH3 |
| Diacetonealcool |
| Diacetone alcohol [UN1148] [Flammable liquid] |
| CAS-123-42-2 |
| Diactone alcool |
| diacetone-alcohol |
| 4-hydroxy-4-methyl-pentan-2-one |
| Diacetonalkohol(german) |
| DAA (CHRIS Code) |
| SCHEMBL28494 |
| 2-Methyl-3-pentanol-4-one |
| Hydroxy-4-methyl-2-pentanone |
| DIACETONE ALCOHOL [MI] |
| 4-hydroxy4-methyl-2-pentanone |
| CHEMBL3182048 |
| DIACETONE ALCOHOL [INCI] |
| 4-hydroxyl-4-methyl-2-pentanone |
| NSC9005 |
| 2-pentanona, 4-hidroxi-4-metil- |
| 4-methyl-4-oxidanyl-pentan-2-one |
| Tox21_201266 |
| Tox21_303479 |
| BBL027463 |
| LMFA12000071 |
| LS-681 |
| MFCD00004471 |
| NA1148 |
| STL146354 |
| AKOS005721167 |
| UN 1148 |
| 4- hydroxy- 4- methylpentan- 2- one |
| 4-Hydroxy-4-methyl-2-pentanone, 99% |
| WLN: QX1 & 1 & 1V1 |
| NCGC00249012-01 |
| NCGC00257419-01 |
| NCGC00258818-01 |
| VS-08543 |
| FT-0624587 |
| H0272 |
| PENTAN-2-ONE, 4-HYDROXY-4-METHYL- |
| EN300-19341 |
| 4-HYDROXY-4-METHYL-2-PENTANONE [HSDB] |
| 4-Hydroksy-4-metyl-2-pentanon (Diacetonalkohol) |
| 4-Hydroxy-4-methyl-pentan-2-on(GERMAN, DUTCH) |
| A805073 |
| Diacetone alcohol [UN1148] [Flammable liquid] |
| Q421486 |
| 4-Hydroxy-4-methyl-2-pentanone, analytical standard |
| DIACETONE ALCOHOL, (FLAMMABLE LIQUID LABEL) |
| J-004939 |
| J-515493 |
| Diacetone alcohol (4-Hydroxy-4-methyl- 2-pentanone) |
| DIACETONE ALCOHOL, (COMBUSTIBLE LIQUID LABEL) |
| F0001-0366 |
| Hydroxy-4-methyl-2-pentanone, 4-; (Diacetone alcohol) |
| InChI=1/C6H12O2/c1-5(7)4-6(2,3)8/h8H,4H2,1-3H |
| Diacetone Alcohol, Pharmaceutical Secondary Standard; Certified Reference Material |
|
There are more than 10 synonyms. If you wish to see them all click here.
|