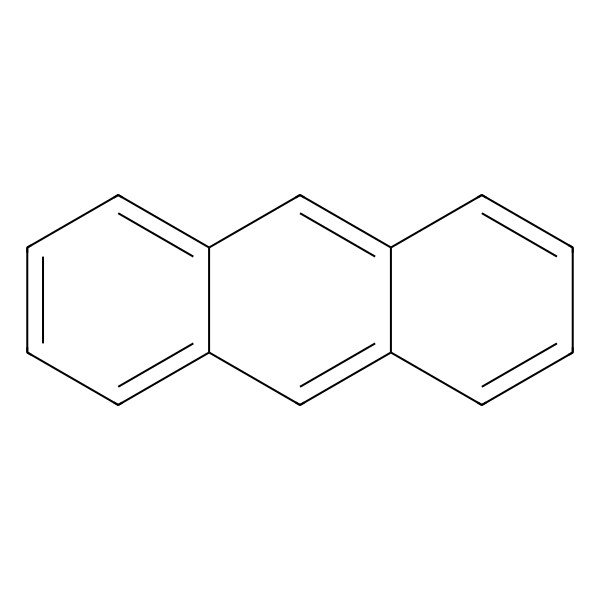
| 120-12-7 |
| Paranaphthalene |
| Anthracin |
| Tetra Olive N2G |
| Anthracen |
| Anthrazen |
| Anthracene, pure |
| Anthracen [German] |
| CCRIS 767 |
| HSDB 702 |
| CHEBI:35298 |
| NSC 7958 |
| EINECS 204-371-1 |
| antraceno |
| Acen |
| p-Naphthalene |
| AI3-00155 |
| UNII-EH46A1TLD7 |
| EH46A1TLD7 |
| Deuterated anthracene |
| DTXSID0023878 |
| Sterilite hop defoliant |
| NSC7958 |
| Coal tar pitch volatiles:anthracene |
| NSC-7958 |
| Tetraoliven2G |
| DTXCID203878 |
| WLN: L C666J |
| Anthracene, labeled with deuterium |
| EC 204-371-1 |
| Coal tar pitch volatiles: anthracene |
| MFCD00001240 |
| NCGC00163972-01 |
| ANTHRACENE (IARC) |
| ANTHRACENE [IARC] |
| C14315 |
| Anthracene, analytical standard |
| Anthraxcene |
| 54261-80-2 |
| AN3 |
| CAS-120-12-7 |
| acene |
| acenes |
| antracene |
| Anthracne |
| Azen |
| 9-Anthracene |
| ANTHRACENE (1,2,3,4,5,6,7,8,9,10-D10) |
| Anthracene technical |
| Anthracene, Reagent |
| HRACENE |
| PARANAPTHALENE |
| Anthracene, Practical |
| ATH (CHRIS Code) |
| ANTHRACENE [MI] |
| ANTHRACENE [HSDB] |
| Epitope ID:119716 |
| A89200_ALDRICH |
| 40076_SUPELCO |
| 48567_SUPELCO |
| 48647_SUPELCO |
| 331481_ALDRICH |
| 46051_RIEDEL |
| CHEMBL333179 |
| Anthracene, puriss., 99.0% |
| 10580_FLUKA |
| Anthracene, reagent grade, 97% |
| CHEBI:35297 |
| Anthracene, zone-refined, >=99% |
| 141062_SIAL |
| Anthracene, ReagentPlus(R), 99% |
| Anthracene (purified by sublimation) |
| Tox21_202226 |
| Tox21_300014 |
| Anthracene, sublimed grade, >=99% |
| BDBM50060894 |
| LS-627 |
| STK398386 |
| AKOS000119970 |
| Anthracene polycyclic aromatic compound |
| AC-5799 |
| (9,10-Dihydroanthracene-9,10-diyl) |
| Anthracene 10 microg/mL in Acetonitrile |
| NCGC00163972-02 |
| NCGC00163972-03 |
| NCGC00254204-01 |
| NCGC00259775-01 |
| Anthracene 100 microg/mL in Acetonitrile |
| AS-14635 |
| A0405 |
| A0495 |
| A0992 |
| FT-0622409 |
| FT-0662238 |
| EN300-18023 |
| J3.643.785E |
| Anthracene Zone Refined (number of passes:30) |
| D88363 |
| Anthracene, vial of 250 mg, analytical standard |
| Anthracene, Zone Refined (number of passes:30) |
| A804437 |
| Q422152 |
| J-200085 |
| Anthracene, certified reference material, TraceCERT(R) |
| Anthracene, suitable for scintillation, >=99.0% (GC) |
| F0001-0328 |
| InChI=1/C14H10/c1-2-6-12-10-14-8-4-3-7-13(14)9-11(12)5-1/h1-10 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|