Strophanthidin
| Internal ID | 38bac85d-6bbe-49ce-a899-a2f523913773 |
| Taxonomy | Lipids and lipid-like molecules > Steroids and steroid derivatives > Steroid lactones > Cardenolides and derivatives |
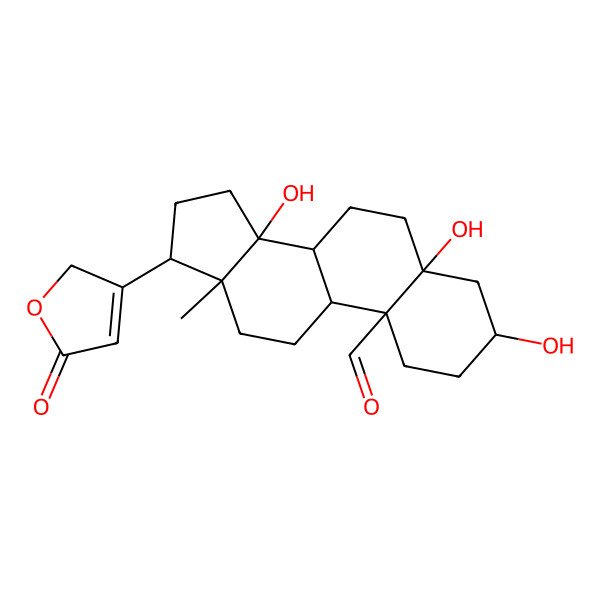
| IUPAC Name | (3S,5S,8R,9S,10S,13R,14S,17R)-3,5,14-trihydroxy-13-methyl-17-(5-oxo-2H-furan-3-yl)-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-10-carbaldehyde |
| SMILES (Canonical) | CC12CCC3C(C1(CCC2C4=CC(=O)OC4)O)CCC5(C3(CCC(C5)O)C=O)O |
| SMILES (Isomeric) | C[C@]12CC[C@H]3[C@H]([C@]1(CC[C@@H]2C4=CC(=O)OC4)O)CC[C@]5([C@@]3(CC[C@@H](C5)O)C=O)O |
| InChI | InChI=1S/C23H32O6/c1-20-6-3-17-18(4-8-22(27)11-15(25)2-7-21(17,22)13-24)23(20,28)9-5-16(20)14-10-19(26)29-12-14/h10,13,15-18,25,27-28H,2-9,11-12H2,1H3/t15-,16+,17-,18+,20+,21-,22-,23-/m0/s1 |
| InChI Key | ODJLBQGVINUMMR-HZXDTFASSA-N |
| Popularity | 1,438 references in papers |
| Molecular Formula | C23H32O6 |
| Molecular Weight | 404.50 g/mol |
| Exact Mass | 404.21988874 g/mol |
| Topological Polar Surface Area (TPSA) | 104.00 Ų |
| XlogP | 0.60 |
| Strophanthidine |
| Convallatoxigenin |
| 66-28-4 |
| Corchsularin |
| k-Strophanthidin |
| Corchorgenin |
| Corchorin |
| Erysimupicrone |
| Corchoside A aglycon |
| Strophanthidin K |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL4096 | P04637 | Cellular tumor antigen p53 |
501.2 nM 501.2 nM 501.2 nM |
Potency Potency Potency |
via Super-PRED
via CMAUP via Super-PRED |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha |
501.2 nM |
Potency |
via Super-PRED
|
| CHEMBL1293277 | O15118 | Niemann-Pick C1 protein |
1778.3 nM |
Potency |
via CMAUP
|
| CHEMBL2362980 | Q06710 | Paired box protein Pax-8 |
2220 nM < 260 nM |
AC50 AC50 |
via CMAUP
via CMAUP |
| CHEMBL1293294 | P51151 | Ras-related protein Rab-9A |
1412.5 nM |
Potency |
via CMAUP
|
| CHEMBL1963 | P16473 | Thyroid stimulating hormone receptor |
25.1 nM |
Potency |
via Super-PRED
|
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL1994 | P08235 | Mineralocorticoid receptor | 95.91% | 100.00% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 94.88% | 91.11% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 93.88% | 96.09% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 93.04% | 95.56% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 92.25% | 94.45% |
| CHEMBL4026 | P40763 | Signal transducer and activator of transcription 3 | 91.80% | 82.69% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 89.15% | 86.33% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 87.80% | 97.09% |
| CHEMBL4208 | P20618 | Proteasome component C5 | 85.73% | 90.00% |
| CHEMBL1871 | P10275 | Androgen Receptor | 85.31% | 96.43% |
| CHEMBL5608 | Q16288 | NT-3 growth factor receptor | 84.26% | 95.89% |
| CHEMBL3492 | P49721 | Proteasome Macropain subunit | 83.95% | 90.24% |
| CHEMBL2581 | P07339 | Cathepsin D | 82.15% | 98.95% |
| CHEMBL253 | P34972 | Cannabinoid CB2 receptor | 81.69% | 97.25% |
| CHEMBL5697 | Q9GZT9 | Egl nine homolog 1 | 81.59% | 93.40% |
| CHEMBL226 | P30542 | Adenosine A1 receptor | 81.54% | 95.93% |
| CHEMBL3351 | Q13085 | Acetyl-CoA carboxylase 1 | 81.39% | 93.04% |
| CHEMBL4478 | Q00975 | Voltage-gated N-type calcium channel alpha-1B subunit | 81.24% | 97.14% |
| CHEMBL3922 | P50579 | Methionine aminopeptidase 2 | 80.86% | 97.28% |
| CHEMBL3746 | P80365 | 11-beta-hydroxysteroid dehydrogenase 2 | 80.43% | 94.78% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| PubChem | 6185 |
| NPASS | NPC72772 |
| ChEMBL | CHEMBL111743 |
| LOTUS | LTS0023973 |
| wikiData | Q1718068 |