| 5986-55-0 |
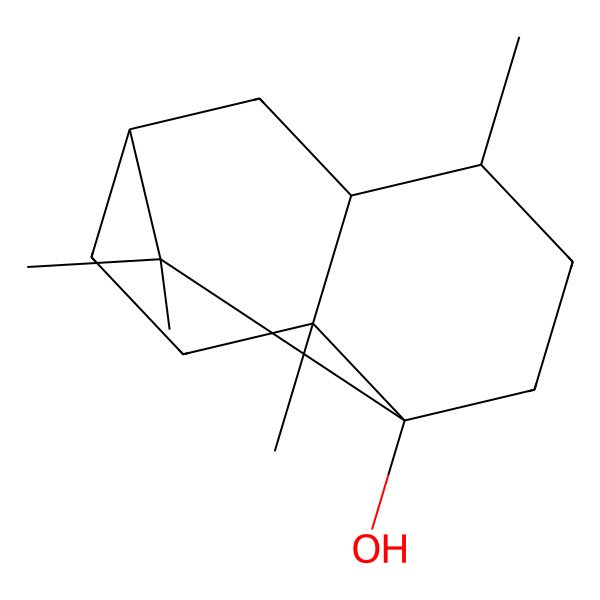
| Patchoulol |
| patchouli camphor |
| Patchoulic alcohol |
| patchoulanol |
| (-)-patchouli alcohol |
| (-)-patchoulol |
| UNII-HHH8CPR1M2 |
| HHH8CPR1M2 |
| solid |
| EINECS 227-807-2 |
| 1,6-Methanonaphthalen-1(2H)-ol, octahydro-4,8a,9,9-tetramethyl-, (1R,4S,4aS,6R,8aS)- |
| (1R,3R,6S,7S,8S)-2,2,6,8-tetramethyltricyclo[5.3.1.03,8]undecan-3-ol |
| (1R,4S,4aS,6R,8aS)-4,8a,9,9-tetramethyloctahydro-1,6-methanonaphthalen-1(2H)-ol |
| (1r,4s,4as,6r,8as)-octahydro-4,8a,9,9-tetramethyl-1,6-methanonaphthalen-1(2h)-ol |
| [1R-(1alpha,4beta,4aalpha,6beta,8aalpha)]-octahydro-4,8a,9,9-tetramethyl-1,6-methano-1(2H)-naphthol |
| Patchoulialkohol |
| (-)-(1R,3R,6S,7S,8S)-2,2,6,8-tetramethyltricyclo[5.3.1.0(3,8)]undecan-3-ol |
| 1,6-Methanonaphthalen-1.beta.(2H)-ol, 3,4,4a.beta.,5,6.beta.,7,8,8a-octahydro-4.alpha.,8a.beta.,9,9-tetramethyl- |
| crystallization |
| pacholol |
| patcholol |
| patschuli |
| white |
| Healingwood |
| healing wood |
| Patchoulol Crist |
| Patchouli alkohol |
| Patchouli-alcohol |
| (1R-(1alpha,4beta,4aalpha,6beta,8aalpha))-Octahydro-4,8a,9,9-tetramethyl-1,6-methano-1(2H)-naphthol |
| Patchouli Oil Terpeneless |
| SCHEMBL108115 |
| PATCHOULI ALCOHOL [MI] |
| CHEBI:7940 |
| CHEMBL4588682 |
| DTXSID9052266 |
| GGHMUJBZYLPWFD-CUZKYEQNSA-N |
| octahydro-4,8a,9,9-tetramethyl- |
| (1R - (1a,4b,4aa,6b,8aa)) - octahydro - 4,8a,9,9 - tetramethyl - 1,6 - methano - 1(2H) - naphthol |
| [1R-(1alpha,4beta,4aalpha,6beta,8aalpha)]-Octahydro-4,8a,9,9-tetramethyl-1,6-methanonaphthalen-1(2H)-ol |
| 1,6-Methanonaphthalen-1(2H)-ol, octahydro-4,8a,9,9-tetramethyl-, (1.alpha.,4.beta.,4a.alpha.,6.beta.,8a.alpha.)- |
| 1,6-METHANONAPHTHALEN-1(2H)-OL, OCTAHYDRO-4,8A,9,9-TETRAMETHYL-, (1R-(1.ALPHA.,4.BETA.,4A.ALPHA.,6.BETA.,8A.ALPHA.))- |
| 1,6-Methanonaphthalen-1(2H)-ol, octahydro-4,8a,9,9-tetramethyl-, (1R-(1alpha,4beta,4aalpha,6beta,8aalpha))- |
| 1,6-Methanonaphthalen-1(2H)-ol, octahydro-4,8a,9,9-tetramethyl-,[1R-(1.alpha.,4.beta.,4a.alpha.,6.beta.,8a.alpha.)]- |
| HY-N0207 |
| s3778 |
| AKOS015840173 |
| AKOS016011426 |
| CCG-266740 |
| NCGC00482601-01 |
| Octahydro-Tetramethyl-Methano-1-Naphthol |
| AC-34015 |
| CS-0008254 |
| FT-0686673 |
| Q7144372 |
| (1R-(1a,4b,4aa,6b,8aa))-Octahydro-4,8a,9,9-tetramethyl-1,6-methano-1(2H)-naphthol 1,6-Methanonaphthalene-1(2H)-ol |
| [1R-1alpha,4beta,4a-alpha,6beta* 4,8a,9,9-Tetramethyloctahydro-1,6-methanonaphthalen-1(2H)-ol |
|
There are more than 10 synonyms. If you wish to see them all click here.
|