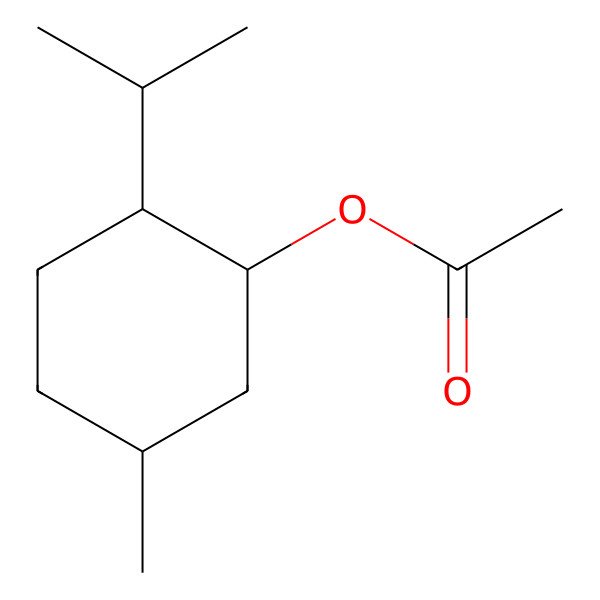
| (-)-Menthyl acetate |
| (1R)-(-)-Menthyl acetate |
| 2623-23-6 |
| l-Menthol acetate |
| dl-Menthyl acetate |
| 89-48-5 |
| Menthyl acetate, dl- |
| (-)-Acetoxy-p-menthane |
| L-p-Menth-3-yl acetate |
| (1R,2S,5R)-rel-2-Isopropyl-5-methylcyclohexyl acetate |
| (dl)-Menthyl acetate |
| (+/-)-Menthyl acetate |
| [(1R,2S,5R)-5-methyl-2-propan-2-ylcyclohexyl] acetate |
| NSC-3722 |
| Menthyl acetate, (-)- |
| LF3LEI45OH |
| UNII-W8C5F4H1OA |
| W8C5F4H1OA |
| p-Menth-3-yl acetate, dl- |
| CHEBI:104 |
| NSC3722 |
| Acetic acid, L- |
| NSC52970 |
| EINECS 201-911-8 |
| EINECS 249-409-8 |
| NSC-52970 |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R,2S,5R)-rel- |
| Menthol, acetate, cis-1,3,trans-1,4- |
| (+-)-Menthol acetate |
| (+-)-Menthyl acetate |
| l-Menthyl acetate (natural) |
| dl-5-Methyl-2-(1-methylethyl)cyclohexyl acetate |
| 4-06-00-00153 (Beilstein Handbook Reference) |
| FEMA No. 2668 |
| Cyclohexan-1-ol, acetate, L- |
| rel-(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl acetate |
| (1R,2S,5R)-5-methyl-2-(propan-2-yl)cyclohexyl acetate |
| (1R,2S,5R)-5-Methyl-2-(1-methylethyl)cyclohexanol 1-acetate |
| NSC 3722 |
| EINECS 220-076-0 |
| 5-Methyl-2-(1-methylethyl)cyclohexyl acetate, (1alpha,2beta,5alpha)- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, 1-acetate, (1R,2S,5R)- |
| NSC 52970 |
| L - menthyl acetate |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, 1-acetate, (1R,2S,5R)-rel- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1alpha,2beta,5alpha)- |
| BRN 2208505 |
| WLN: L6TJ AY1&1 BOV1 D1 -L |
| Menthol, acetate, (1R,3R,4S)-(-)- |
| UNII-LF3LEI45OH |
| (5R)-2-Isopropyl-5-methylcyclohexyl acetate |
| ACETIC ACID, p-MENTH-3-YL ESTER, l- |
| Cyclohexan-1-ol, 2-isopropyl-5-methyl-, acetate, L- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R,2S,5R)- |
| l-Menthyl acetate (1alpha,2beta,5alpha) |
| L-?Menthyl acetate |
| Nat.Menthyl Acetate |
| laevo-menthyl acetate |
| EC 201-911-8 |
| (-)-?Menthyl acetate |
| MENTHYL ACETATE, L- |
| SCHEMBL57397 |
| MENTHYL ACETATE [MI] |
| MENTHYL ACETATE [INCI] |
| MENTHYL ACETATE, RACEMIC |
| P- MENTH-3-YL ACETATE |
| CHEMBL2236867 |
| DTXSID7051472 |
| DTXSID9041817 |
| MENTHYL ACETATE [MART.] |
| (+/-)-MENTHOL ACETATE |
| L-MENTHYL ACETATE [FHFI] |
| CYCLOHEXANOL, 5-METHYL-2-(1-METHYLETHYL)-, ACETATE, (1.ALPHA.,2.BETA.,5.ALPHA.)- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R-(1alpha,2beta,5alpha))- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1theta-(1alpha,2beta,5alpha))- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, [1R-(1.alpha.,2.beta.,5.alpha.)]- |
| HY-N7132 |
| (-)-MENTHYL ACETATE [FCC] |
| MENTHYL ACETATE, (+/-)- |
| MFCD00001482 |
| MFCD00063644 |
| s5597 |
| (1R)-(-)-Menthyl acetate, 98% |
| MENTHYL ACETATE RACEMATE [MI] |
| AKOS015913189 |
| CCG-266575 |
| CS-W010530 |
| LS-2351 |
| MENTHYL ACETATE, RACEMIC [FCC] |
| AS-71989 |
| FEMA NO. 2668, (-)- |
| A1107 |
| M3399 |
| FEMA NO. 2668, (+/-)- |
| C09870 |
| D88378 |
| F77849 |
| (1R)-(-)-Menthyl acetate, analytical standard |
| L-Menthyl acetate, natural, >=98%, FCC, FG |
| A861167 |
| (1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl acetate |
| Q6817577 |
| W-107187 |
| Cyclohexanol,5-methyl-2-(1-methylethyl)-,1-acetate |
|
There are more than 10 synonyms. If you wish to see them all click here.
|