| 6902-77-8 |
| (+)-Genipin |
| CHEBI:5298 |
| UNII-A3V2NE52YG |
| A3V2NE52YG |
| ST080860 |
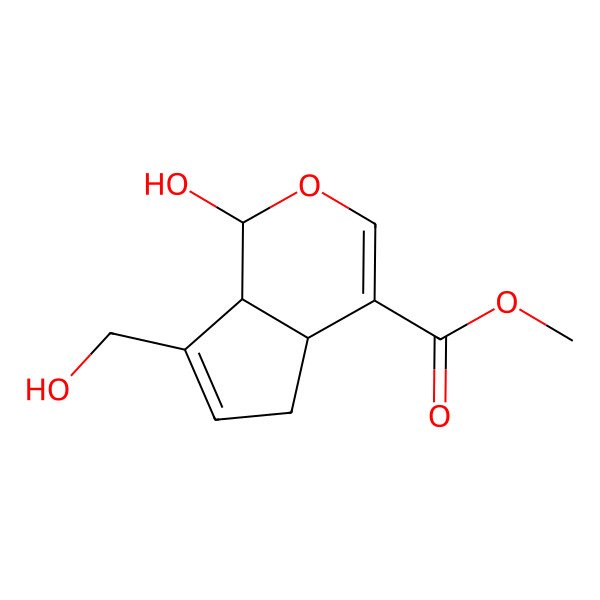
| methyl (1R,4aS,7aS)-1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate |
| C09780 |
| (1R,4aS,7aS)-methyl 1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate |
| methyl (1R,4aS,7aS)-1-hydroxy-7-(hydroxymethyl)-1H,4aH,5H,7aH-cyclopenta[c]pyran-4-carboxylate |
| 1,4a,5,7a-Tetrahydro-1-hydroxy-7-(hydroxymethyl)-cyclopenta(c)pyran-4-carboxylic acid methyl ester |
| AC1L9CSN |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1,4A,5,7A-TETRAHYDRO-1-HYDROXY-7-(HYDROXYMETHYL)-, METHYL ESTER, (1R,4AS,7AS)- |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1,4A,5,7A-TETRAHYDRO-1-HYDROXY-7-(HYDROXYMETHYL)-, METHYL ESTER, (1R-(1.ALPHA.,4A.ALPHA.,7A.ALPHA.))- |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1,4A.ALPHA.,5,7A.ALPHA.-TETRAHYDRO-1-HYDROXY-7-(HYDROXYMETHYL)-, METHYL ESTER |
| Cyclopenta[c]pyran-4-carboxylic acid, 1,4a,5,7a-tetrahydro-1-hydroxy-7-(hydroxymethyl)-, methyl ester, (1R,4aS,7aS)- |
| Cyclopenta[c]pyran-4-carboxylic acid, 1,4a,5,7a-tetrahydro-1-hydroxy-7-(hydroxymethyl)-, methyl ester, [1R-(1.alpha.,4a.alpha.,7a.alpha.)]- |
| Cyclopenta[c]pyran-4-carboxylic acid, 1,4a.alpha.,5,7a.alpha.-tetrahydro-1-hydroxy-7-(hydroxymethyl)-, methyl ester |
| MFCD00888600 |
| SureCN34249 |
| SCHEMBL34249 |
| MLS006010198 |
| CHEMBL459016 |
| DTXSID30894999 |
| AZKVWQKMDGGDSV-BCMRRPTOSA-N |
| HMS3261H13 |
| Cyclopenta(c)pyran-4-carboxylic acid, 1,4a-alpha,5,7a-alpha-tetrahydro-1-hydroxy-7-(hydroxymethyl)-, methyl ester |
| EX-A4263 |
| Tox21_500516 |
| AM1140 |
| BDBM50565452 |
| Genipin, >=98% (HPLC), powder |
| s2412 |
| AKOS015851487 |
| AC-8847 |
| BCP9000722 |
| CCG-221820 |
| CS-1096 |
| CS-O-30595 |
| LP00516 |
| SDCCGSBI-0633724.P001 |
| NCGC00186010-01 |
| NCGC00186010-03 |
| NCGC00186010-10 |
| NCGC00261201-01 |
| AS-18947 |
| HY-17389 |
| Methyl (1S,2R,6S)-2-Hydroxy-9-(hydroxymethyl)-3-oxabicyclo[4.3.0]nona-4,8-diene-5-carboxylate |
| SMR001456266 |
| G0458 |
| AB01566854_01 |
| EN300-7409283 |
| A836320 |
| J-521411 |
| Q1463401 |
| BRD-K28824103-001-04-4 |
| Genipin is known as an aglycone dervied from Geniposide. |
| Z2044761865 |
| (1R,4aS,7aS)-1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylic acid methyl ester |
| (1R,4aS,7aS)-1-Hydroxy-7-hydroxymethyl-1,4a,5,7a-tetrahydro-cyclopenta[c]pyran-4-carboxylic acid methyl ester |
| 1202641-87-9 |
| Cyclopenta[c]pyran-4-carboxylic acid, 1,4a,5,7a-tetrahydro-1-hydroxy-7-(hydroxymethyl)-, methylester, (1R,4aS,7aS)- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|