| .beta.-D-Glucopyranoside, methyl |
| Methyl .beta.-galactoside |
| Methyl mannoside |
| .beta.-D-Altropyranoside, methyl |
| METHYL-A-D-ALTROPYRANOSIDE |
| .beta.-Methyl-D-galactopyranoside |
| methyl-D-glucoside |
| .beta.-D-Galactopyranose methyl glycoside |
| Methylmannoside |
| Glucopyranoside, .alpha.-D- |
| a-methylglucoside |
| Methyl .beta.-D-galactopyranoside |
| .beta.-D-Galactopyranoside, methyl |
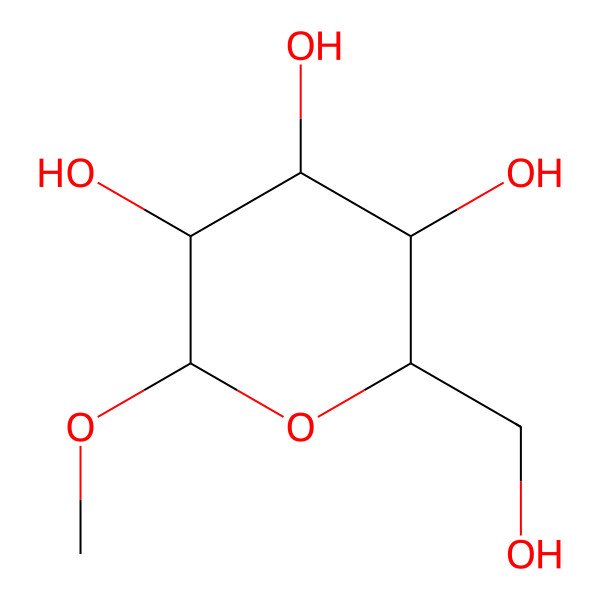
| (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-methoxyoxane-3,4,5-triol |
| (D3)METHYL A-D-GLUCOSIDE |
| Methyl .beta.-D-glucoside |
| Methyl .alpha.-D-mannoside |
| METHYLGLUCOSIDE, ALPHA |
| .alpha.-Methyl-D-galactoside |
| Methyl .alpha.-D-galactoside |
| Methyl .beta.-D-glucopyranoside |
| C7H14O6 |
| Methyl .alpha.-D-mannopyranoside |
| .alpha.-Methyl-D-galactopyranoside |
| MFCD00006602 |
| MFCD00063262 |
| NSC-33684 |
| NSC-33685 |
| 1-O-Methyl-.beta.-D-glucopyranoside |
| Glucopyranoside, methyl, .alpha.-D- |
| Galactopyranoside, methyl, .beta.-D- |
| NSC-102101 |
| NSC-214092 |
| Methyl D-galactoside |
| .beta.-Methylglucoside |
| Methyl |A-D-glucoside |
| Methyl-.beta.-galactoside |
| Methyl ?-D-mannopyranoside |
| NCIOpen2_001794 |
| 2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol |
| Mannopyranoside, .alpha.-D- |
| MLS001049132 |
| .alpha.-Methyl mannopyranoside |
| SCHEMBL1430362 |
| Methyl-.beta.-D-thiogalactoside |
| SCHEMBL19738683 |
| SCHEMBL21847893 |
| HOVAGTYPODGVJG-UHFFFAOYSA- |
| DTXSID60859172 |
| NSC1225 |
| NSC3817 |
| HOVAGTYPODGVJG-UHFFFAOYSA-N |
| HMS2268J22 |
| Methyl |A-D-Glucoside Hemihydrate |
| 25281-48-5 |
| NSC-1225 |
| NSC-3817 |
| NSC33684 |
| NSC33685 |
| 1-O-METHYL-BETA-D-GLUCOSIDE |
| NSC102101 |
| NSC163490 |
| NSC214092 |
| NSC227937 |
| NSC403457 |
| NSC403949 |
| 1-O-METHYL-BETA-D-GALACTOSIDE |
| AKOS006227638 |
| NSC-163490 |
| NSC-227937 |
| NSC-403457 |
| NSC-403949 |
| SB44902 |
| SB45069 |
| Methyl ?-D-Galactopyranoside Monohydrate |
| NCGC00246215-01 |
| NCGC00246215-02 |
| 688007-20-7 |
| Mannopyranoside, 1-O-methyl-, alpha-D- |
| SMR000386958 |
| SY066776 |
| SY076496 |
| .alpha.-D-Galactopyranose methyl glycoside |
| 1-O-METHYL-ALPHA-D-GLUCOSIDE HYDRATE |
| FT-0628701 |
| FT-0628704 |
| FT-0628705 |
| FT-0628855 |
| FT-0628856 |
| FT-0628857 |
| FT-0628867 |
| FT-0693585 |
| Methyl .beta.-D-galactopyranoside, .beta.-D- |
| AC-907/25014385 |
| 03AFED88-DA24-4BCE-B79F-84CAF2B36351 |
| Methyl .beta.-D-galactopyranoside, methyl, .beta.-D- |
| InChI=1/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|