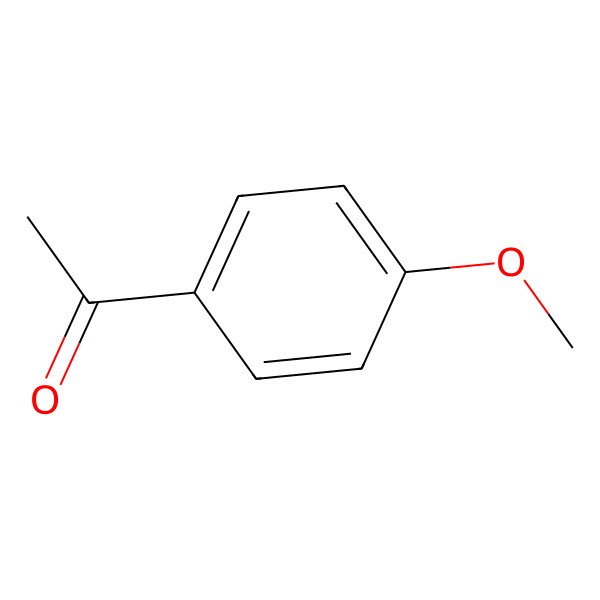
| 100-06-1 |
| 4-Methoxyacetophenone |
| 4-Acetylanisole |
| 1-(4-Methoxyphenyl)ethanone |
| Acetanisole |
| p-Methoxyacetophenone |
| Novatone |
| Linarodin |
| Vananote |
| Ethanone, 1-(4-methoxyphenyl)- |
| 1-(4-methoxyphenyl)ethan-1-one |
| P-ACETYLANISOLE |
| 4-Methoxyphenyl methyl ketone |
| Acetophenone, 4'-methoxy- |
| p-Methoxyphenyl methyl ketone |
| p-methoxy acetophenone |
| Methyl p-methoxyphenyl ketone |
| 4-Methoxyacetofenon |
| Methyl 4-methoxyphenyl ketone |
| FEMA No. 2005 |
| Anisyl, p-, methyl ketone |
| 4-Methoxyacetofenon [Czech] |
| p-Metoxyacetophenone |
| NSC 209523 |
| 4-methoxy acetophenone |
| 4-Methoxy-acetophenone |
| para-Methoxyacetophenone |
| 4'-methoxy-acetophenone |
| EINECS 202-815-9 |
| UNII-0IRH2BR587 |
| 1-(4-methoxyphenyl)-ethanone |
| AI3-00227 |
| 0IRH2BR587 |
| CHEMBL401912 |
| DTXSID2044347 |
| CHEBI:86567 |
| 1-(4-methoxyphenyl)ethanone-OCD3 |
| NSC-5601 |
| NSC-209523 |
| EC 202-815-9 |
| WLN: 1VR DO1 |
| para-Acetanisole |
| 4-METHOXYACETOPHENONE (D3) |
| 1804943-43-8 |
| MFCD00008745 |
| acetylanisole |
| 4-Acetoanisole |
| p-methoxyactophenone |
| paramethoxyacetophenone |
| 4'-methoxyacetophenon |
| p -methoxyacetophenone |
| p-methoxy-acetophenone |
| 4 -methoxyacetophenone |
| Acetophenone, p-methoxy- |
| 4' - methoxyacetophenone |
| ACETANISOLE [FCC] |
| ACETANISOLE [FHFI] |
| bmse010024 |
| SCHEMBL41285 |
| 1-(4-methoxy-phenyl)ethanone |
| 1-(4-methoxyphenyl) ethanone |
| 4'-Methoxyacetophenone, 99% |
| Etanona, 1-(4-metoxifenil)- |
| 1-(4-Methoxy-phenyl)-ethanone |
| 1-(4-Methoxyphenyl)ethanone # |
| DTXCID0024347 |
| SCHEMBL12015229 |
| FEMA 2005 |
| HSDB 8319 |
| 1-(4-methoxyphenyl)-1-ethanone |
| 1-[4-(methyloxy)phenyl]ethanone |
| NSC5601 |
| Acetanisole, >=98%, FCC, FG |
| AMY21966 |
| STR00157 |
| Tox21_300687 |
| BDBM50376209 |
| NSC209523 |
| STK498196 |
| AKOS000119536 |
| LS-2536 |
| PS-3395 |
| NCGC00188270-01 |
| NCGC00188270-02 |
| NCGC00254595-01 |
| AC-10916 |
| CAS-100-06-1 |
| 4'-Methoxyacetophenone, analytical standard |
| BB 0258870 |
| CS-0008322 |
| FT-0618906 |
| M0105 |
| EN300-16104 |
| D71251 |
| 4'-Methoxyacetophenone, purum, >=99.0% (GC) |
| AB-131/40174083 |
| Q229995 |
| W-108978 |
| Z53832960 |
| F0001-0007 |
| InChI=1/C9H10O2/c1-7(10)8-3-5-9(11-2)6-4-8/h3-6H,1-2H |
| 4-Acetylanisole 4'-Methoxyacetophenone 1-(4-Methoxyphenyl)ethanone Acetanisole Novatone 4-Acetylanisole Linarodin Vananote Acetophenone, 4'-methoxy- Ethanone, 1-(4-methoxyphenyl)- p-Methoxyacetophenone |
| O9F |
|
There are more than 10 synonyms. If you wish to see them all click here.
|