| 96-76-4 |
| Antioxidant No. 33 |
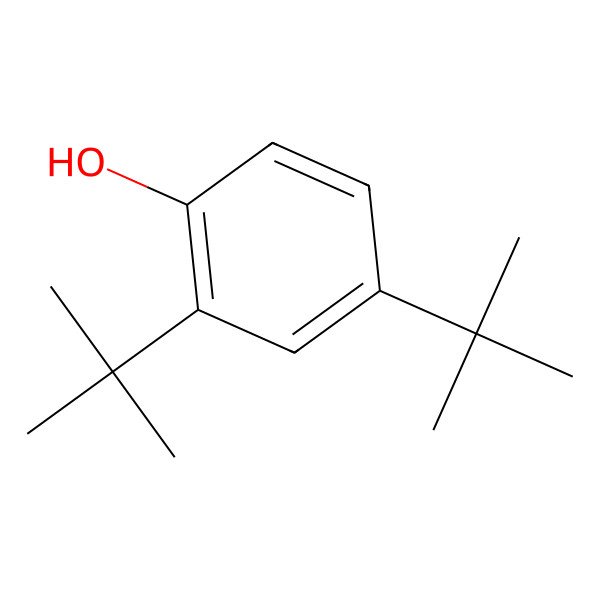
| 2,4-DI-T-BUTYLPHENOL |
| 1-Hydroxy-2,4-di-tert-butylbenzene |
| Prodox 146 |
| Phenol, 2,4-bis(1,1-dimethylethyl)- |
| Phenol, 2,4-di-tert-butyl- |
| 2,4-bis(tert-butyl)phenol |
| 2,4-ditert-butylphenol |
| Prodox 146A-85X |
| 2,4-Bis(1,1-dimethylethyl)phenol |
| 2,4-tert-butylphenol |
| MFCD00008828 |
| NSC 174502 |
| 2,4-Di-tert-butylphenol-d18 |
| 2,4-ditert-butyl-phenol |
| EINECS 202-532-0 |
| UNII-FOB94G6HZT |
| FOB94G6HZT |
| 2,4-di-tert-butyl phenol |
| 2,4-Di-tert-butyl-phenol |
| Phenol, 2,4-di(1,1-dimethylethyl)- |
| BRN 1910383 |
| 2,4-di~{tert}-butylphenol |
| CHEMBL29873 |
| DTXSID2026602 |
| 2,4-Di-tert-butylhydroxybenzene |
| 2,4-Bis(1,1'-dimethylethyl)phenol |
| 2,4-bis(1,1-dimethylethyl)-phenol |
| NSC-174502 |
| 1246816-88-5 |
| EC 202-532-0 |
| DTXCID606602 |
| CAS-96-76-4 |
| 2,4-bis[1,1,1,3,3,3-hexadeuterio-2-(trideuteriomethyl)propan-2-yl]phenol |
| 2,4-DTBP |
| 2,4-ditertbutylphenol |
| 2,4-di-tertbutylphenol |
| 2,4di-tert-butylphenol |
| ,4-Di-tert-butylphenol |
| 2,4-di-t-butyl-phenol |
| 2,4-di-tert.butylphenol |
| 2,4-di-tertbutyl phenol |
| 2,4-ditert-butyl phenol |
| 2,4-ditertiarybutylphenol |
| Phenol,4-di-tert-butyl- |
| AGIDOL 10 |
| ?2,4-Di-tert-butylphenol |
| 2,4-Di-tert.-butylphenol |
| 2,4-ditertiary-butyl phenol |
| SCHEMBL109921 |
| CHEBI:89188 |
| HSDB 8453 |
| 2,4-Di-tert-butylphenol, 99% |
| BCP24012 |
| Phenol,4-bis(1,1-dimethylethyl)- |
| Tox21_202320 |
| Tox21_300114 |
| BDBM50409544 |
| NSC174502 |
| Phenol,2,4-Bis(1,1-dimethylethyl) |
| 1-Hydroxy-2, 4-di-tert-butylbenzene |
| AKOS003669719 |
| CS-W015305 |
| HY-W014589 |
| NCGC00164059-01 |
| NCGC00164059-02 |
| NCGC00164059-03 |
| NCGC00254167-01 |
| NCGC00259869-01 |
| AS-13983 |
| PD158314 |
| 2,4-Ditert-butylphenol (ACD/Name 4.0) |
| WLN: 1X1&1&R BQ CX1&1&1 |
| LS-104318 |
| D0229 |
| FT-0610222 |
| EN300-20927 |
| E76999 |
| A845633 |
| Q-200191 |
| Q26840829 |
| 2,4-Di-tert-butylphenol 100 microg/mL in Acetonitrile |
| F0001-2302 |
| (2S)-N-[(1S)-2-[[(1S)-2-[[(1S)-2-[[(1S)-2-[[2-[[(1S)-1-[[(1S)-4-amino-1-[(2S)-2-[(2-amino-2-oxo-ethyl)carbamoyl]pyrrolidine-1-carbonyl]-4-oxo-butyl]carbamoyl]-3-methyl-butyl]amino]-2-oxo-ethyl]amino]-1-[(4-hydroxyphenyl)methyl]-2-oxo-ethyl]amino]-1-(hydro |
| InChI=1/C14H22O/c1-13(2,3)10-7-8-12(15)11(9-10)14(4,5)6/h7-9,15H,1-6H |
| UGW |
|
There are more than 10 synonyms. If you wish to see them all click here.
|