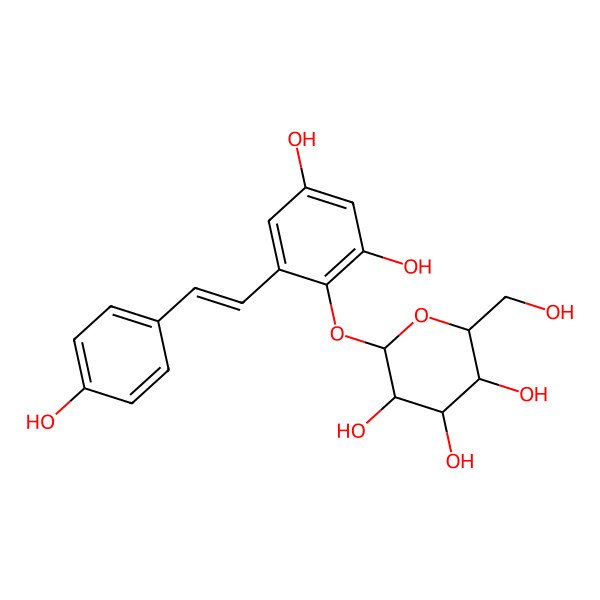
| 2,3,5,4'-Tetrahydroxystilbene 2-O-glucoside |
| 55327-45-2 |
| 2,3,5,4'-Tetrahydroxystilbene-2-O-b-D-glucopyranoside |
| 2,3,5,4-tetrahydroxyl diphenylethylene-2-o-glucoside |
| 54QRI6OKJ5 |
| EH-201 |
| 2,3,4',5-Tetrahydroxystilbene 2-o-D-glucoside |
| UNII-54QRI6OKJ5 |
| CHEMBL460860 |
| (2S,3R,4S,5S,6R)-2-(2,4-dihydroxy-6-((E)-4-hydroxystyryl)phenoxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| (E)-2,3,5,4'-Tetrahydroxy stilbene-2-O-glucoside |
| (2S,3R,4S,5S,6R)-2-[2,4-dihydroxy-6-[(E)-2-(4-hydroxyphenyl)ethenyl]phenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| 2,3,4',5-Tetrahydroxystilbene 2-O-Glucoside |
| 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-D-glucoside |
| 2,3,5,4'-Tetrahydroxyl diphenylethylene-2-O-glucoside |
| beta-D-Glucopyranoside, 2,4-dihydroxy-6-(2-(4-hydroxyphenyl)ethenyl)phenyl |
| 2,4-Dihydroxy-6-((1E)-2-(4-hydroxyphenyl)ethenyl)phenyl beta-D-glucopyranoside |
| beta-D-Glucopyranoside, 2,4-dihydroxy-6-((1E)-2-(4-hydroxyphenyl)ethenyl)phenyl |
| beta-D-Glucopyranoside, 2,4-dihydroxy-6-(2-(4-hydroxyphenyl)ethenyl)phenyl, (E)- |
| 2,3,5,4'-tetrahydroxystilbene-2-o-beta-d-glucopyranoside |
| (2S,3R,4S,5S,6R)-2-[2,4-dihydroxy-6-[(E)-2-(4-hydroxyphenyl)vinyl]phenoxy]-6-(hydroxymethyl)tetrahydropyran-3,4,5-triol |
| 2,3,5,4'-tetrahydroxystilbene 2-O-beta-D-glucoside |
| 2,3,5,4'-TETRAHYDROXYSTILBENE-2-O-BETA-D-GLUCOSIDE (USP-RS) |
| 2,3,5,4'-TETRAHYDROXYSTILBENE-2-O-BETA-D-GLUCOSIDE [USP-RS] |
| tetrahydroxyldiphenylethylene-2-o-glucoside |
| tetrahydroxystilbene glucoside |
| SCHEMBL2688101 |
| SCHEMBL17507729 |
| CHEBI:140850 |
| DTXSID801242061 |
| BDBM50020713 |
| MFCD00238694 |
| s3906 |
| AKOS030530177 |
| AKOS040758637 |
| CCG-268716 |
| tetrahydroxyl diphenylethylene-2-o-gluco |
| NCGC00482790-01 |
| Tetrahydroxystilbene-2-O-??-D-glucoside |
| AC-33974 |
| AS-76404 |
| T3513 |
| 2,3,5,4'-Tetrahydroxystilbene 2-O-glucosid |
| F16062 |
| 2,3,5,4\'-Tetrahydroxystilbene 2-O-glucoside |
| 2,3,5,4'-Tetrahydroxy stilbene-2---D-glucoside |
| 2,3,5,4'-Tetrahydroxystilbene 2-O--D-glucoside |
| A864422 |
| 2,3,5,4'-Tetrahydroxystilbene 2-O-b-D-glucoside |
| 2,3,5,4'-Tetrahydroxystilbene 2-O-fA-D-Glucoside |
| 2,3,5,4'-tetrahydroxystilbene-2-O-?-D-glucoside |
| 2,3,5,4'-Tetrahydroxystilbene-2-O-ss-D-glucoside |
| 2,3,5,4'-Tetrahydroxytoluylene-2-beta-D-glucoside |
| 2-(beta-D-Glucopyranosyloxy)stilbene-3,4',5-triol |
| Q-100633 |
| W-203871 |
| 2,3,5,4-tetrahydroxyl-diphenylethylene-2-o-glucoside |
| (E)-2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside |
| 2,3,5,4'-Tetrahydroxytoluylene-2-beta-D-glucopyranoside |
| 2,3,5,4-Tetrahydroxy stilbene-2-Omicron-beta-D-glucoside |
| trans-2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside |
| (E)-2-(beta-D-Glucopyranosyloxy)-3,4',5-trihydroxystilbene |
| 2,3,5,4'-Tetrahydroxyl-diphenylethylene-2-O-beta-D-glucoside |
| 2,3,5,4'-TETRAHYDROXYSTILBENE-2-O-.BETA.-D-GLUCOSIDE |
| .BETA.-D-GLUCOPYRANOSIDE, 2,4-DIHYDROXY-6-((1E)-2-(4-HYDROXYPHENYL)ETHENYL)PHENYL |
| .BETA.-D-GLUCOPYRANOSIDE, 2,4-DIHYDROXY-6-(2-(4-HYDROXYPHENYL)ETHENYL)PHENYL, (E)- |
| 2,4-DIHYDROXY-6-((1E)-2-(4-HYDROXYPHENYL)ETHENYL)PHENYL .BETA.-D-GLUCOPYRANOSIDE |
| 2,4-dihydroxy-6-[(E)-2-(4-hydroxyphenyl)ethenyl]phenyl beta-D-glucopyranoside |
| B-D-GLUCOPYRANOSIDE,2,4-DIHYDROXY-6-[2-(4-HYDROXYPHENYL)ETHENYL]PHENYL |
| 2,3,5,4'-Tetrahydroxystilbene 2-O-AfAE'A centa' notA inverted exclamation markAfasA'A|AfAE'Adaggeratrade markAfA centA centasA notA em leaderA inverted exclamation mark-D-glucoside |
|
There are more than 10 synonyms. If you wish to see them all click here.
|