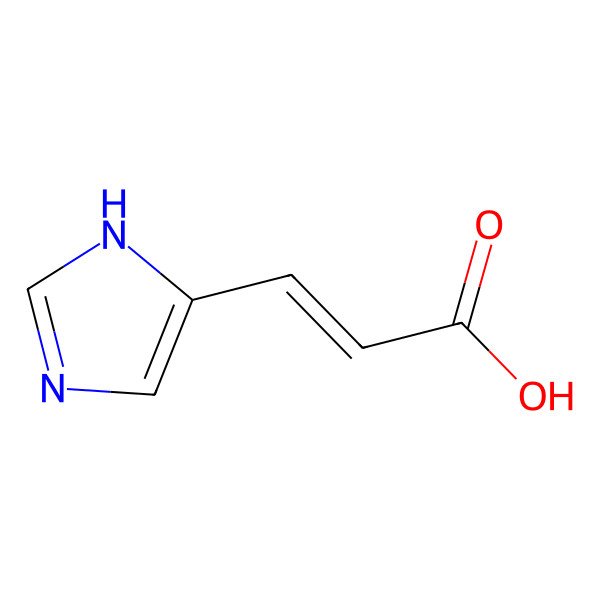
| 4-imidazoleacrylic acid |
| trans-Urocanic acid |
| 104-98-3 |
| 3465-72-3 |
| urocanate |
| Urocaninic acid |
| imidazoleacrylic acid |
| 5-Imidazoleacrylic acid |
| Imidazole-4-acrylic acid |
| (2E)-3-(1H-IMIDAZOL-4-YL)ACRYLIC ACID |
| 3-Imidazol-4-ylacrylic acid |
| (E)-3-(1H-imidazol-5-yl)acrylic acid |
| 3-(1H-Imidazol-4-yl)-2-propenoic acid |
| 3-(1H-imidazol-4-yl)acrylic acid |
| (2E)-3-(1H-imidazol-4-yl)prop-2-enoic acid |
| (E)-3-(1H-imidazol-5-yl)prop-2-enoic acid |
| (E)-3-(1H-Imidazol-4-yl)-2-propenoic acid |
| 2-Propenoic acid, 3-(1H-imidazol-4-yl)- |
| 3-(4-Imidazolyl)acrylic acid |
| (E)-3-(4-Imidazolyl)acrylic acid |
| (E)-Urocanic acid |
| NSC 66357 |
| UNII-G8D26XJJ3B |
| G8D26XJJ3B |
| MLS000069482 |
| DTXSID5041148 |
| CHEBI:30817 |
| 4-IMIDAZOLE ACRYLIC ACID DIHYDRATE |
| 2-Propenoic acid, 3-(1H-imidazol-4-yl)-, (E)- |
| NSC-66357 |
| 2-Propenoic acid, 3-(1H-imidazol-4-yl)-, (2E)- |
| CCRIS 3414 |
| SMR000059098 |
| EINECS 203-258-4 |
| (E)-3-(1H-imidazol-4-yl)prop-2-enoic acid |
| BRN 0081405 |
| 3-imidazol-4-ylacrylsyre |
| 3- imidazol- 4- ylacrylic acid |
| (E)-3-(imidazol-4-yl)propenoate |
| (Z)-Urocanic acid;cis-UCA |
| (e)-3-(1h-imidazol-4-yl)acrylic acid |
| ProtoCure |
| 3-(1H-imidazol-4-yl)prop-2-enoic acid |
| 5-Imidazoleacrylate |
| MFCD00005203 |
| Imidazole-4-acrylate |
| 2-Propenoic acid, 3-(1H-imidazol-5-yl)- |
| Opera_ID_26 |
| UROCANIC ACID, CIS |
| 3-(4-Imidazolyl)acrylate |
| bmse000279 |
| D04CQV |
| Lopac0_001255 |
| SCHEMBL15417 |
| trans-4-imidazoleacrylic acid |
| UROCANIC ACID [INCI] |
| MLS001148240 |
| UROCANIC ACID, TRANS- |
| 4-Imidazoleacrylic acid, 99% |
| 3-(1H-Imidazol-4-yl)acrylate |
| CHEMBL1236602 |
| DTXCID3021148 |
| CHEBI:27248 |
| AMY7015 |
| E-3-(4-imidazolyl)propenoic acid |
| HMS2234N08 |
| HMS3263L12 |
| NSC66357 |
| Tox21_202978 |
| Tox21_501255 |
| BBL027507 |
| MFCD01593677 |
| NSC407934 |
| s9449 |
| STK735136 |
| (E)-3-(imidazol-4-yl)acrylic acid |
| 3-(3H-Imidazol-4-yl)-acrylic acid |
| 3-(1H-Imidazol-4-yl)-2-propenoate |
| AKOS001745677 |
| AKOS005265082 |
| AKOS022488522 |
| (e)-3-(Imidazol-4-yl)propenoic acid |
| CCG-205329 |
| DB01971 |
| HS-0075 |
| LP01255 |
| SDCCGSBI-0051222.P002 |
| (E)-3-(1H-imidazol-5-yl)acrylicacid |
| IMIDAZOLE-4-ACRYLIC ACID, (E)- |
| NCGC00094495-01 |
| NCGC00094495-02 |
| NCGC00094495-03 |
| NCGC00094495-05 |
| NCGC00260524-01 |
| NCGC00261940-01 |
| CAS-104-98-3 |
| LS-78025 |
| 3-Azonia-1H-imidazole-5-acrylic acidanion |
| HY-113008 |
| CS-0059340 |
| EU-0101255 |
| I0002 |
| 1H-ImidaZole-4-acrylic acid (Urocanic acid) |
| 2-Propenoic acid, 3-(1H-imidazole-4-yl)- |
| C00785 |
| E72446 |
| EN300-343465 |
| Q30536 |
| U 7500 |
| (2E)-3-(1H-imidazol-5-yl)prop-2-enoic acid |
| (E)-3-(IMIDAZOL-4-YL)-2-PROPENOIC ACID |
| A936841 |
| Imidazole-4-propene-2-enoic acid [Urocanic acid] |
| SR-01000076189 |
| SR-01000076189-1 |
| W-108797 |
| Q60998685 |
| ProtoCure (emulsion, atopic dermatitis), Biocis Pharma |
| 23A7DA77-EC02-46B9-AA22-514C2B2A62E9 |
| ProtoCure (emulsion, atopic dermatitis/psoriasis), Laurantis |
| Anti-inflammatory (topical emulsion, dermatological disease), BioCis |
| Cis-UCA (topical emulsion, atopic dermatitis/psoriasis), Laurantis |
| ProtoCure (emulsion, atopic dermatitis/psoriasis), Biocis Pharma |
| Cis-UCA (topical emulsion, atopic dermatitis/psoriasis), BioCis Pharma |
| 3-(1h-imidazol-4-yl)-2-propenoicaci ;3-(1h-imidazol-4-yl)-2-propenoicacid |
| Cis-urocanic acid (topical emulsion, atopic dermatitis/psoriasis), BioCis Pharma |
|
There are more than 10 synonyms. If you wish to see them all click here.
|