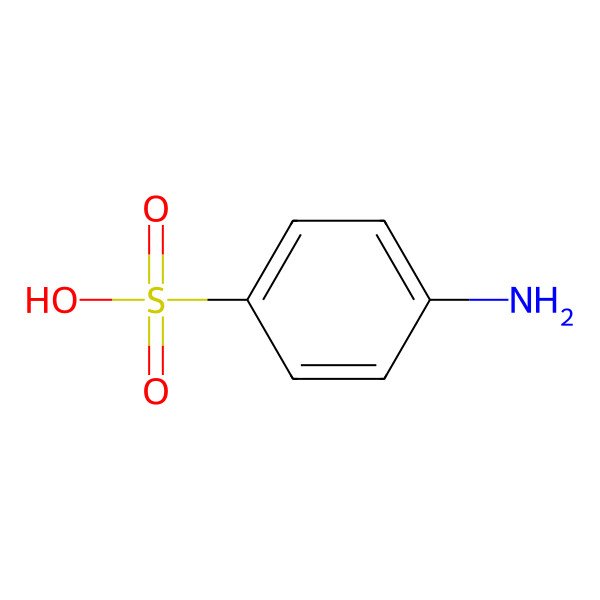
| 4-Aminobenzenesulfonic acid |
| 121-57-3 |
| Sulphanilic acid |
| p-Aminobenzenesulfonic acid |
| Aniline-4-sulfonic acid |
| Sulfanilsaeure |
| Aniline-p-sulfonic acid |
| Aniline-p-sulphonic acid |
| Benzenesulfonic acid, 4-amino- |
| p-Aminophenylsulfonic acid |
| p-Anilinesulfonic acid |
| Kyselina sulfanilova |
| 4-Sulfoaniline |
| 4-Sulfanilic acid |
| CHEBI:27500 |
| NSC 7170 |
| CCRIS 4576 |
| p-sulfoaniline |
| Sulfanilsaeure [German] |
| HSDB 5590 |
| EINECS 204-482-5 |
| p-Sulfoaniline-d4 |
| Kyselina sulfanilova [Czech] |
| 4-aminobenzene-1-sulfonic acid |
| AI3-15414 |
| UNII-434Z8C2635 |
| NSC-7170 |
| 4-aminophenylsulfonic acid |
| C6H7NO3S |
| p-aminobenzene sulfonic acid |
| 4-aminobenzene sulfonic acid |
| 1-aminobenzene-4-sulfonic acid |
| 434Z8C2635 |
| CHEMBL1566888 |
| DTXSID6024464 |
| EC 204-482-5 |
| MFCD00007886 |
| 71949-32-1 |
| 97675-28-0 |
| SULFANILIC ACID (USP-RS) |
| SULFANILIC ACID [USP-RS] |
| MESALAZINE IMPURITY O (EP IMPURITY) |
| MESALAZINE IMPURITY O [EP IMPURITY] |
| SULFADIAZINE IMPURITY B (EP IMPURITY) |
| SULFADIAZINE IMPURITY B [EP IMPURITY] |
| SUFANILIC ACID |
| SULFADIMIDINE IMPURITY F (EP IMPURITY) |
| SULFADIMIDINE IMPURITY F [EP IMPURITY] |
| 4-Aminobenzenesulphonic Acid (Sulphanilic Acid) |
| SULFADIMETHOXINE IMPURITY D (EP IMPURITY) |
| SULFADIMETHOXINE IMPURITY D [EP IMPURITY] |
| SULFAMETHOXAZOLE IMPURITY D (EP IMPURITY) |
| SULFAMETHOXAZOLE IMPURITY D [EP IMPURITY] |
| P-SULFANILIC ACID |
| ANILINE P-SULFONIC ACID |
| SULFADIMETHOXINE SODIUM FOR VETERINARY USE IMPURITY D (EP IMPURITY) |
| SULFADIMETHOXINE SODIUM FOR VETERINARY USE IMPURITY D [EP IMPURITY] |
| Sulfanilic acid; |
| Acide sulfanilique |
| p-sulphanilic acid |
| 4-AMINOBENZENESULPHONIC ACID |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with 5,5'-oxybis(1,3-benzenediol), sodium salt |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with 5,5'-oxybis[1,3-benzenediol], sodium salt |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with diazotized 4-((4-aminophenyl)azo)benzenesulfonic acid and resorcinol |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with diazotized 4-[(4-aminophenyl)azo]benzenesulfonic acid and resorcinol |
| Sulfanilic acid, 99% |
| Sulfanilic Acid Anhydrous |
| bmse000726 |
| Epitope ID:122241 |
| p-aminobenzenesulphonic acid |
| 4-aminobenzene-sulfonic acid |
| SCHEMBL24407 |
| SULFANILIC ACID [MI] |
| 4-Amino benzenesulphonic acid |
| Sulfanilic acid, >=99.0% |
| SULFANILIC ACID [HSDB] |
| 4-amino-1-benzenesulfonic acid |
| DTXCID604464 |
| Sulfanilic acid, 98%, tech. |
| NSC7170 |
| PARA-ANILINE SULFONIC ACID |
| Sulfanilate Zinc (sulfanilic acid) |
| EINECS 276-203-5 |
| EINECS 285-231-7 |
| EINECS 286-256-6 |
| EINECS 290-689-6 |
| EINECS 290-691-7 |
| EINECS 305-460-9 |
| EINECS 307-598-5 |
| Nitrate Reagent B, for microbiology |
| BBL011603 |
| BDBM50443531 |
| LS-650 |
| STK661383 |
| Sulfanilic acid, ACS reagent, 99% |
| AKOS000118732 |
| AM91128 |
| NCGC00090886-01 |
| NCGC00090886-02 |
| 85049-60-1 |
| 85203-66-3 |
| 90218-16-9 |
| 90218-18-1 |
| 94552-15-5 |
| AC-12565 |
| VS-02984 |
| Sulfanilic Acid [for Biochemical Research] |
| Sulfanilic acid, p.a., 98.0-102.0% |
| FT-0674701 |
| S0120 |
| U0107 |
| EN300-19269 |
| C06335 |
| E80415 |
| Sulfanilic acid, Vetec(TM) reagent grade, 99% |
| Q253746 |
| Sulfanilic acid, puriss. p.a., >=99.0% (T) |
| J-004536 |
| 4-Aminobenzenesulfonic acid 100 microg/mL in Methanol |
| Sulfanilic acid, JIS special grade, 99.0-100.5% |
| F0001-0346 |
| Z104473374 |
| Sulfanilic acid, United States Pharmacopeia (USP) Reference Standard |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with m-phenylenediamine |
| Sulfanilic acid, 99.0-100.5%, suitable for determination of nitroxide |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with C.I. Natural Yellow 11 |
| Benzenesulfonic acid,4-amino-,diazotized,coupled with dyer's mulberry extract |
| InChI=1/C6H7NO3S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H,8,9,10 |
| Sulfanilic Acid, Pharmaceutical Secondary Standard; Certified Reference Material |
| Benzenesulfonic acid, 4-amino-, diazotized, coupled with 4-methyl-1,3-benzenediamine and m-phenylenediamine, sodium salt |
| Benzenesulfonic acid,4-amino-,diazotized,coupled with 5,5'-oxybis[1,3-benzenediol],sodium salt |
|
There are more than 10 synonyms. If you wish to see them all click here.
|