| 57-92-1 |
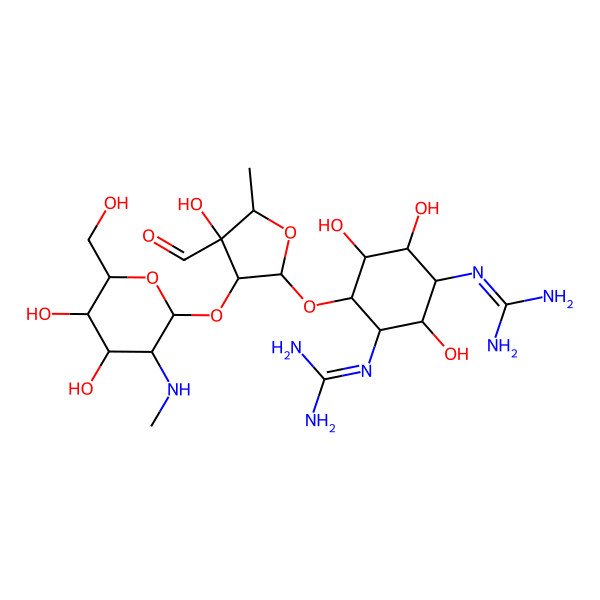
| Streptomycin A |
| Strepcen |
| Gerox |
| Streptomycine |
| Agrimycin |
| Agrept |
| Neodiestreptopab |
| Streptomicina |
| Streptomyzin |
| CHEBI:17076 |
| Streptomycin sulphate |
| Estreptomicina |
| Streptomycinum |
| Hokko-mycin |
| Caswell No. 804 |
| NSC-14083 |
| streomycin |
| Chemform |
| Streptomycin sulfate |
| UNII-Y45QSO73OB |
| Y45QSO73OB |
| CCRIS 5730 |
| HSDB 1768 |
| EINECS 200-355-3 |
| EPA Pesticide Chemical Code 006306 |
| BRN 0074498 |
| Streptomycin A sulfate |
| 2,4-Diguanidino-3,5,6-trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-alpha-L-glucopyranosyl)-3-C-formyl-beta-L-lyxopentanofuranoside |
| 2-[(1R,2R,3S,4R,5R,6S)-3-(diaminomethylideneamino)-4-[(2R,3R,4R,5S)-3-[(2S,3S,4S,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy-2,5,6-trihydroxycyclohexyl]guanidine |
| 4-18-00-07540 (Beilstein Handbook Reference) |
| streptomycin- |
| Streptomycin (INN) |
| D-Streptamine, O-2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl-(1-2)-O-5-deoxy-3-C-formyl-alpha-L-lyxofuranosyl-(1-4)-N,N'-bis(aminoiminomethyl)- |
| STREPTOMYCIN [INN] |
| Streptomycin, Sulfate Salt |
| STREPTOMYCIN (MART.) |
| STREPTOMYCIN [MART.] |
| 2,4-Diguanidino-3,5,6-trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-alpha-glucopyranosyl)-3-formylpentofuranoside |
| Streptomycin Sesquisulfate Hydrate |
| NSC 14083 |
| Streptomycin (TN) |
| Liposomal Streptomycin |
| [2-deoxy-2-(dimethylamino)-alpha-L-glucopyranosyl]-(1->2)-[5-deoxy-3-C-formyl-alpha-L-lyxofuranosyl]-(1->4)-{N',N'''-[(1,3,5/2,4,6)-2,4,5,6-tetrahydroxycyclohexane-1,3-diyl]diguanidine} |
| D-Streptamine, O-2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl-(1.fwdarw.2)-O-5-deoxy-3-C-formyl-alpha-L-lyxofuranosyl-(1.fwdarw.4)-N,N'-bis(aminoiminomethyl)- |
| C21-H39-N7-O12 |
| NCGC00159339-02 |
| Streptomycin,(S) |
| 1-[(1S,2R,3R,4S,5R,6R)-2-[(2R,3R,4R,5S)-3-[(2S,3S,4S,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)tetrahydropyran-2-yl]oxy-4-formyl-4-hydroxy-5-methyl-tetrahydrofuran-2-yl]oxy-5-guanidino-3,4,6-trihydroxy-cyclohexyl]guanidine |
| SRY |
| NSC14083 |
| D-Streptamine, O-2-deoxy-2-(methylamino) -?-L-glucopyranosyl-(1.fwdarw.2)-O-5-deoxy-3 -C-formyl-?-L-lyxofuranosyl-(1.fwdarw.4)-N,N '-bis(aminoiminomethyl)- |
| D-Streptamine, O-2-deoxy-2-(methylamino)-.alpha.-L-glucopyranosyl-(1.fwdarw.2)-O-5-deoxy-3-C-formyl-.alpha.-L-lyxofuranosyl-(1.fwdarw.4)-N,N'-bis(aminoiminomethyl)- |
| D-Streptamine, O-2-deoxy-2-(methylamino)-.alpha.-L-glucopyranosyl-(1?2)-O-5-deoxy-3-C-formyl-.alpha.-L-lyxofuranosyl-(1?4)-N,N'-bis(aminoiminomethyl)- |
| D-Streptamine, O-2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl-(12)-O-5-deoxy-3-C-formyl-alpha-L-lyxofuranosyl-(14)-N,N'-bis(aminoiminomethyl)- |
| D-Streptamino, O-2-desoxi-2-(metilamino)-alfa-L-glucopiranosil-(1>2)-O-5-deoxi-3-C-formil-alfa-L-lixofuranosil (14 )-N1, N3-bis (aminoiminometil)- |
| STREPTOMYCIN [MI] |
| Epitope ID:224553 |
| STREPTOMYCIN [HSDB] |
| SCHEMBL3276 |
| STREPTOMYCIN [VANDF] |
| STREPTOMYCIN [WHO-DD] |
| CHEMBL372795 |
| DTXSID4023597 |
| GTPL10923 |
| A07AA04 |
| J01GA01 |
| STREPTOMYCIN [GREEN BOOK] |
| UCSJYZPVAKXKNQ-HZYVHMACSA-N |
| -(hydroxymethyl)-3-(methylamino) |
| Streptomycin Sesquisulphate Hydrate |
| HY-B1906 |
| N,N'''-[(1R,2R,3S,4R,5R,6S)-4-({5-deoxy-2-O-[2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl]-3-C-formyl-alpha-L-lyxofuranosyl}oxy)-2,5,6-trihydroxycyclohexane-1,3-diyl]diguanidine |
| N,N'''-[(1R,2R,3S,4R,5R,6S)-4-{5-deoxy-2-O-[2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl]-3-C-formyl-alpha-L-lyxofuranosyloxy}-2,5,6-trihydroxycyclohexane-1,3-diyl]diguanidine |
| O-2-Deoxy-2-(methylamino)-.alpha.-L-glucopyranosyl-(1->2)-O-5-deoxy-3-C-formyl-.alpha.-L-lyxofuranosyl-(1->4)-N,N'-bis(aminoiminomethyl)-D-streptamine and Liposome |
| BDBM50103513 |
| AKOS015969759 |
| AM84920 |
| DB01082 |
| AS-35042 |
| tetrahydro-2H-pyran-2-yloxy)-4-formyl-4 |
| -hydroxy-5-methyltetrahydrofuran-2-yloxy)- |
| LS-187084 |
| CS-0013964 |
| C00413 |
| D08531 |
| 3-((2S,3S,4S,5R,6S)-4,5-dihydroxy-6 |
| AB00443813_02 |
| EN300-7481099 |
| 2,5,6-trihydroxycyclohexane-1,3-diyl)diguanidine |
| Q192717 |
| BRD-K07166362-330-02-8 |
| 1,-((1R,2R,3S,4R,5R,6S)-4-((2R,3R,4R,5S)- |
| 1-[(1S,2R,3R,4S,5R,6R)-3-carbamimidamido-6-{[(2R,3R,4R,5S)-3-{[(2S,3S,4S,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy}-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy}-2,4,5-trihydroxycyclohexyl]guanidine |
| D-Streptamine, O-2-deoxy-2-(methylamino)-.alpha.-L-glucopyranosyl-(1->2)-O-5-deoxy-3-C-formyl-.alpha.-L-lyxofuranosyl-(1->4)-N,N'-bis(aminoiminomethyl)- |
| N-[(1R,2R,3S,4R,5R,6S)-3-carbamimidamido-4-{[(2R,3R,4R,5S)-3-{[(2S,3S,4S,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy}-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy}-2,5,6-trihydroxycyclohexyl]guanidine |
|
There are more than 10 synonyms. If you wish to see them all click here.
|