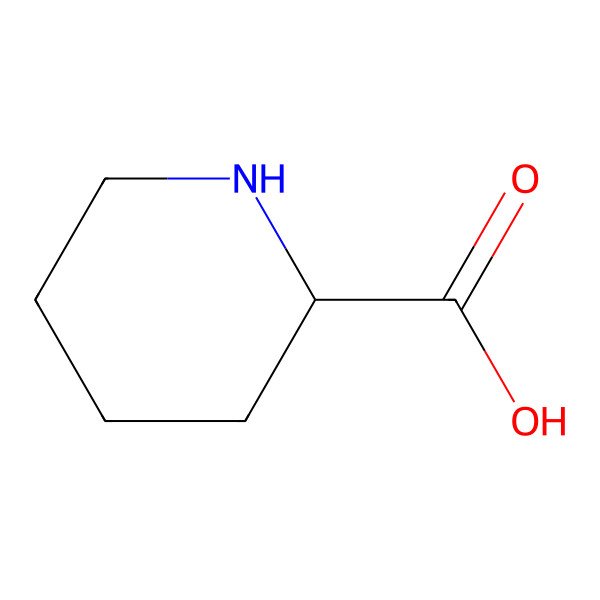
| DL-Pipecolinic acid |
| 535-75-1 |
| Pipecolic acid |
| Pipecolinic acid |
| 2-PIPERIDINECARBOXYLIC ACID |
| 4043-87-2 |
| pipecolate |
| Homoproline |
| DL-Pipecolic acid |
| Dihydrobaikiane |
| Piperolinic acid |
| L-PipecolicAcid |
| Hexahydropicolinic acid |
| l(-)-pipecolinic acid |
| DL-Homoproline |
| Acide pipecolique |
| 2-Carboxypiperidine |
| MFCD00064347 |
| (+/-)-Pipecolic acid |
| Hexahydro-2-picolinic acid |
| 2-Pipecolinic acid |
| Pipecolic acid, L- |
| Acide piperidine-carboxylique-2 |
| 83680-83-5 |
| .alpha.-Pipecolinic acid |
| (+/-)-2-Piperidinecarboxylic Acid |
| (+/-)-Pipecolinic acid |
| H254GW7PVV |
| Pipecolic acid, (S)-(-)- |
| CHEBI:17964 |
| 2-Piperidinecarboxylic acid, (S)- |
| 2-Pyridine(carboxylic acid-13C1) |
| (R)-(+)-2-Piperidinecarboxylic acid |
| (+/-)-Piperidine-2-carboxylic acid |
| NSC-17125 |
| 2-Piperidinecarboxylic acid, (A+/-)- |
| DL-2-PIPERIDINE-D9-CARBOXYLIC ACID |
| 2-Piperidinecarboxylate |
| DL-PipecolicAcid |
| Acide pipecolique [French] |
| 2-piperidinic acid |
| Pipecolic acid, L-(-)- |
| EINECS 208-616-3 |
| EINECS 223-737-1 |
| NSC 17125 |
| (D)-(+)-Pipercolicacid |
| UNII-H254GW7PVV |
| Pipecolinate |
| Pipecolinicacid |
| Piperolinate |
| Acide piperidine-carboxylique-2 [French] |
| a-Pipecolinate |
| DL-Pipecolinate |
| DL-Pipecolate |
| MFCD00064346 |
| NSC-93089 |
| h-dl-homopro-oh |
| alpha-Pipecolinate |
| h-dl-hopro-oh |
| Hexahydropicolinate |
| a-Pipecolinic acid |
| (+/-)-Pipecolate |
| alpha-Pipecolinic acid |
| (+/-)-Pipecolinate |
| Hexahydro-2-picolinate |
| Pipecolic acid free base |
| (+/-) pipecolic acid |
| Pipecolinic acid, 98% |
| DL-2-Piperidinecarboxylate |
| 2-Piperidinecarboxylic acidd |
| 2-Piperidinylcarboxylic acid |
| 2-piperidine carboxylic acid |
| 2-piperidine-carboxylic acid |
| NCIOpen2_001582 |
| PIPECOLIC ACID [MI] |
| piperidine-6-carboxylic acid |
| SCHEMBL22016 |
| (RS)-2-Piperidinecarboxylate |
| 2-Piperidinecarboxylic acid # |
| dl-2-piperidinecarboxylic acid |
| CHEMBL308408 |
| (+/-)-2-Piperidinecarboxylate |
| HXEACLLIILLPRG-UHFFFAOYSA- |
| ()-Piperidine-2-carboxylic acid |
| DL-Pipecolic acid;H-DL-Pip-OH |
| DTXSID40862144 |
| (RS)-2-Piperidinecarboxylic acid |
| CS-B1313 |
| HY-Y0669 |
| NSC17125 |
| NSC93089 |
| PIPECOLIC ACID DL-FORM [MI] |
| AC-691 |
| s6322 |
| AKOS000120345 |
| AKOS016050399 |
| (.+/-.)-2-Piperidinecarboxylic acid |
| AB00554 |
| AC-2835 |
| AM90343 |
| PS-5851 |
| 2-Piperidinecarboxylic acid, (.+/-.)- |
| SY002975 |
| SY003075 |
| SY003076 |
| A7793 |
| FT-0601107 |
| FT-0601108 |
| FT-0613361 |
| FT-0625508 |
| FT-0650084 |
| FT-0673913 |
| P0442 |
| EN300-20680 |
| A23910 |
| Q7197255 |
| W-106353 |
| W-111701 |
| F2191-0193 |
| Z104479780 |
| ( inverted exclamation markA)-Piperidine-2-carboxylic acid |
| InChI=1/C6H11NO2/c8-6(9)5-3-1-2-4-7-5/h5,7H,1-4H2,(H,8,9) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|