| 1504-74-1 |
| 60125-24-8 |
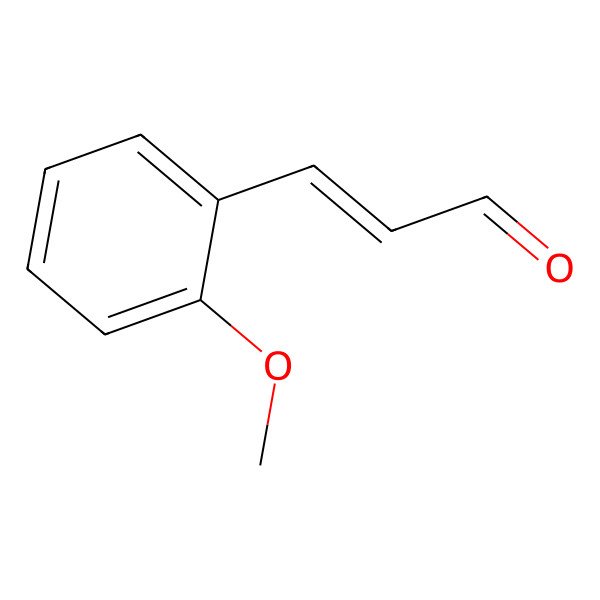
| O-METHOXYCINNAMALDEHYDE |
| (E)-3-(2-Methoxyphenyl)acrylaldehyde |
| o-Methoxycinnamic aldehyde |
| (E)-2-methoxycinnamaldehyde |
| 2'-Methoxycinnamaldehyde |
| 2-Propenal, 3-(2-methoxyphenyl)- |
| Cinnamaldehyde, o-methoxy- |
| Cassiastearoptene |
| o-Methoxy cinnamaldehyde |
| 3-(2-Methoxyphenyl)acrylaldehyde |
| (2E)-3-(2-Methoxyphenyl)Prop-2-Enal |
| 3-O-Methoxyphenyl-2-propenal |
| beta-(o-Methoxyphenyl)acrolein |
| O-Methoxycinnamaldehyde, (E)- |
| (E)-3-(2-methoxyphenyl)prop-2-enal |
| o-Methoxycinnamicaldehyde crystals |
| 3-(2-Methoxyphenyl)-2-propenal |
| UNII-3GG2B2MUHB |
| 2-Methoxycinnamic aldehyde |
| 3GG2B2MUHB |
| Ortho-Methoxycinnamaldehyde |
| trans-2-Methoxycinnamaldehyde |
| FEMA No. 3181 |
| CCRIS 3196 |
| O-Methoxyphenyl acrolein, beta- |
| FEMA 3181 |
| o-Methoxycinnamaldehyde (natural) |
| 2-Methoxy Cinnamaldehyde |
| 2-propenal, 3-(2-methoxyphenyl)-, (2E)- |
| Methoxycinnamaldehyde, o- |
| EINECS 216-131-3 |
| NSC 114599 |
| (2E)-3-(2-methoxyphenyl)acrylaldehyde |
| BRN 2436856 |
| (2E)-3-(2-Methoxyphenyl)-2-propenal |
| CHEMBL83159 |
| UNII-4940G3R6HE |
| Ortho methoxy cinnamic aldehyde |
| DTXSID4047206 |
| TRANS-O-METHOXYCINNAMALDEHYDE |
| O-METHOXYCINNAMALDEHYDE, (2E)- |
| 2-08-00-00149 (Beilstein Handbook Reference) |
| 2-PROPENAL, 3-(2-METHOXYPHENYL)-, (E)- |
| 2-Methoxycinnamaldehyde, predominantly trans |
| 2' - methoxycinnamaldehyde |
| 3-(2-methoxyphenyl)prop-2-enal |
| DTXSID2025551 |
| .beta.-(o-Methoxyphenyl)acrolein |
| MFCD00007001 |
| 2-Methoxycimnamaldehyde |
| O-Methoxy-Cinnamaldehyde |
| O-Methoxy-Cinnamicaldehyde |
| (E)-O-methoxycinnamaldehyde |
| 2-Methoxybenzeneacrylaldehyde |
| beta-O-Methoxyphenyl acrolein |
| DTXCID405551 |
| beta -(O-methoxyphenyl)acrolein |
| AMY3534 |
| DTXCID90809802 |
| CHEBI:180713 |
| 4940G3R6HE |
| Tox21_202885 |
| Tox21_303635 |
| 3-(2-Methoxyphenyl)-2-propenal # |
| BDBM50240642 |
| LS-879 |
| s9482 |
| (E)-3-(2-Methoxy-phenyl)-propenal |
| AKOS000120956 |
| o-Methoxycinnamaldehyde, >=96%, FG |
| HY-W046353 |
| 3-(2-Methoxyphenyl)-(2E)-2-propenal |
| NCGC00256699-01 |
| NCGC00260431-01 |
| AS-15726 |
| CAS-1504-74-1 |
| CAS-60125-24-8 |
| 2-Methoxycinnamaldehyde, natural, 98%, FG |
| CS-0065992 |
| CS-0201507 |
| EN300-19948 |
| PK04_181244 |
| O10862 |
| EN300-6729490 |
| 2-Methoxycinnamaldehyde, predominantly trans, 96% |
| A809049 |
| A869043 |
| W-108075 |
| BRD-K20078988-001-01-1 |
| Q27257183 |
| Z2573655488 |
| 2'-METHOXYCINNAMALDEHYDE (CONSTITUENT OF CINNAMOMUM CASSIA BARK) |
| 2'-METHOXYCINNAMALDEHYDE (CONSTITUENT OF CINNAMOMUM VERUM BARK) |
| (E)-3-(2-methoxyphenyl)prop-2-enal;2-Methoxycinnamaldehyde, predominantly trans |
| 2'-METHOXYCINNAMALDEHYDE (CONSTITUENT OF CINNAMOMUM CASSIA BARK) [DSC] |
| 2'-METHOXYCINNAMALDEHYDE (CONSTITUENT OF CINNAMOMUM VERUM BARK) [DSC] |
| InChI=1/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|