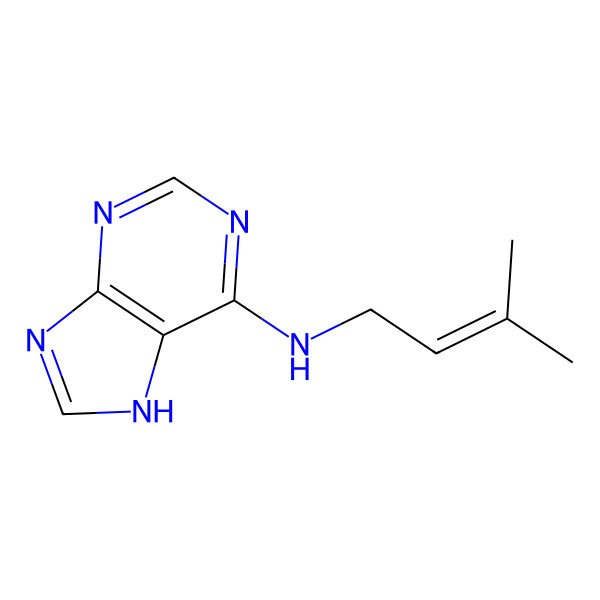
| Isopentenyladenine |
| N-(3-METHYLBUT-2-EN-1-YL)-9H-PURIN-6-AMINE |
| N6-(2-Isopentenyl)adenine |
| N6-Isopentenyladenine |
| 6-(gamma,gamma-Dimethylallylamino)purine |
| Isopentenyl adenine |
| IPADE |
| N6-Dimethylallyladenine |
| N-(3-methylbut-2-enyl)-7H-purin-6-amine |
| N6-(2-Isopentenyl)-adenine |
| Enadenine |
| 1H-Purin-6-amine, N-(3-methyl-2-butenyl)- |
| N(6)-(delta(2)-Isopentenyl)adenine |
| N6-(delta2-Isopentenyl)adenine |
| N(6)-dimethylallyladenine |
| N-(3-Methyl-2-butenyl)adenine |
| N6-(3-Methyl-2-butenyl)adenine |
| N6-(delta2-Isopentenyl)-adenine |
| Adenine, N-(3-methyl-2-butenyl)- |
| 6-((3-Methyl-2-butenyl)amino)purine |
| N6-(2-lsopentenyl)adenine |
| N-(3-Methyl-2-butenyl)-1H-purin-6-amine |
| 6-isopentenyladenine |
| NSC 106958 |
| N6-(3-Methylbut-2-enyl)adenine |
| 6-(|A,|A-Dimethylallylamino)purine |
| NSC106958 |
| NSC-106958 |
| 6-(3,3-DIMETHYLALLYLAMINO)PURINE |
| 2ip |
| CHEBI:17660 |
| N-(.DELTA.2-Isopentenyl)adenine |
| V500BB256Z |
| N6-(.DELTA.2-Isopentenyl)adenine |
| 6-[(3-Methyl-2-butenyl)amino]purine |
| N6-(.DELTA.2-Isopentenyl)aminopurine |
| 6-(3-methyl-2-buten-1-ylamino)purine |
| N-(.gamma.,.gamma.-Dimethylallyl)adenine |
| 6-(.gamma.,.gamma.-Dimethylallylamino)purine |
| n-isopentenyladenine |
| N-(delta2-Isopentenyl)adenine |
| N6-(delta2-Isopentenyl)aminopurine |
| N-(gamma,gamma-Dimethylallyl)adenine |
| UNII-V500BB256Z |
| 2exm |
| BRYOKININ |
| (3-methylbut-2-enyl)purin-6-ylamine |
| N-(3-methylbut-2-enyl)-9H-purin-6-amine |
| NCIStruc1_000935 |
| NCIStruc2_000921 |
| TimTec1_003281 |
| N6-Delta2-isopentenyladenine |
| Oprea1_344117 |
| SCHEMBL459276 |
| CHEMBL476189 |
| 6-(,-Dimethylallylamino)purine |
| 6-(y,y-Dimethylallylamino)purine |
| DTXSID10178325 |
| N-6-(3,3-Dimethylallyl)adenine |
| HMS1543F03 |
| HMS3886C18 |
| N6-(delta 2-isopentenyl) adenine |
| N(6)-(Delta2-isopentenyl)adenine |
| N6-.DELTA.2-Isopentenyl)adenine |
| CCG-38046 |
| MFCD00132998 |
| NCGC00014084 |
| NCI106958 |
| s5690 |
| AKOS005257639 |
| AKOS015914857 |
| N-6-( Delta 2-Isopentenyl)-adenine |
| DB08768 |
| NCGC00014084-02 |
| NCGC00097193-01 |
| 3-methylbut-2-enyl(7H-purin-6-yl)amine |
| AC-15608 |
| AC-32452 |
| AS-17517 |
| NCI60_000165 |
| 6-[(3-methylbut-2-en-1-yl)amino]purine |
| N(6)-(.DELTA.2-Isopentenyl)aminopurine |
| HY-112103 |
| N-(3-methyl-2-butenyl)-9H-purin-6-amine |
| CS-0043352 |
| FT-0629731 |
| 6-N-(3-METHYLBUT-2-ENYLAMINO)PURINE |
| A12318 |
| C04083 |
| D-4760 |
| N-(3-Methyl-2-butenyl)-1H-purin-6-amine # |
| N-(3-methylbut-2-en-1-yl)-7H-purin-6-amine |
| A816825 |
| Q569031 |
| (3-methyl-but-2-enyl)-(7(9)H-purin-6-yl)-amine |
| BRD-K62158429-001-01-7 |
| 9H-PURIN-6-AMINE, N-(3-METHYL-2-BUTEN-1-YL)- |
| N6-(2-Isopentenyl)-adenine;6-(gamma,gamma-Dimethylallylamino)purine |
| 6-(gamma,gamma-Dimethylallylamino)purine, BioReagent, plant cell culture tested, >=90% |
| 6-(gamma,gamma-Dimethylallylamino)purine, BioReagent, plant cell culture tested, >=98.5% |
| 6-(gamma,gamma-Dimethylallylamino)purine, BioReagent, plant cell culture tested, 1 mg/mL |
| 6-(gamma,gamma-Dimethylallylamino)purine, Vetec(TM) reagent grade, >=98.5% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|