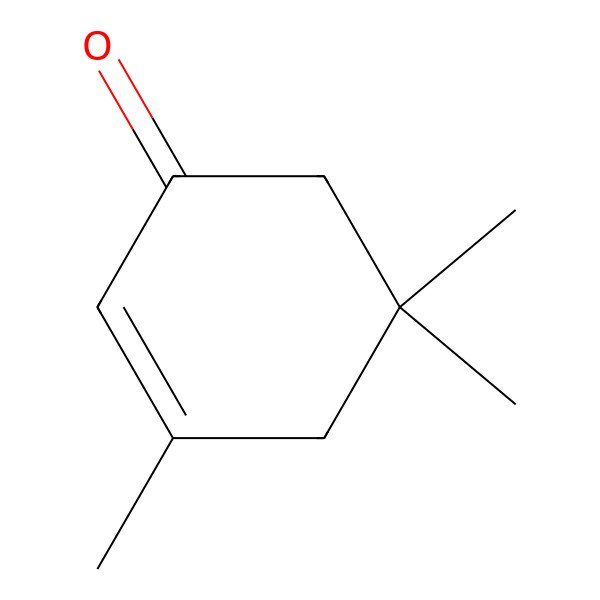
| 78-59-1 |
| Isoacetophorone |
| 3,5,5-Trimethylcyclohex-2-en-1-one |
| Isoforone |
| Isooctopherone |
| Isoforon |
| Izoforon |
| 3,5,5-Trimethyl-2-cyclohexen-1-one |
| 3,5,5-Trimethylcyclohex-2-enone |
| 2-Cyclohexen-1-one, 3,5,5-trimethyl- |
| Isophoron |
| alpha-Isophorone |
| .alpha.-Isophoron |
| 1,1,3-Trimethyl-3-cyclohexene-5-one |
| 3,5,5-Trimethyl-2-cyclohexenone |
| Izoforon [Polish] |
| .alpha.-Isophorone |
| NCI-C55618 |
| Isoforone [Italian] |
| Caswell No. 506 |
| 3,5,5-Trimethyl-2-cyclohexen-1-on |
| FEMA No. 3553 |
| 3,5,5-Trimetil-2-cicloesen-1-one |
| Isophorone, 97% |
| NSC 403657 |
| CCRIS 1353 |
| HSDB 619 |
| EINECS 201-126-0 |
| UNII-2BR99VR6WA |
| EPA Pesticide Chemical Code 047401 |
| 3,5,5-Trimethyl-2-cyclohexene-1-one |
| BRN 1280721 |
| 2BR99VR6WA |
| 3,5,5-Trimethylcyclohexenone |
| AI3-00046 |
| 3,5,5-Trimethylcyclohexen one |
| DTXSID8020759 |
| CHEBI:34800 |
| 3,5,5-Trimetil-2-cicloesen-1-one [Italian] |
| NSC4881 |
| 3,5,5-Trimethylcyclohexen-2-one-1 |
| 3,5,5-Trimethyl-2-cyclohexen-1-on [German, Dutch] |
| 1,5,5-Trimethyl-1-cyclohexen-3-one |
| 3,3,5-Trimethyl-2-cyclohexen-1-one |
| NSC-403657 |
| EC 201-126-0 |
| 4-07-00-00165 (Beilstein Handbook Reference) |
| DTXCID40759 |
| 3,5-Trimethyl-2-cyclohexenone |
| 3,5-Trimetil-2-cicloesen-1-one |
| 3,5-Trimethyl-2-cyclohexen-1-one |
| 1,3-Trimethyl-3-cyclohexene-5-one |
| 3,5-Trimethyl-2-cyclohexene-1-one |
| WLN: L6V BUTJ C1 D1 D1 |
| 2-Cyclohexen-1-one,5,5-trimethyl- |
| CAS-78-59-1 |
| ISOPHORONE, REAG |
| 3,5-Trimethyl-2-cyclohexen-1-on (GERMAN, DUTCH) |
| Isoforona |
| a-Isophorone |
| alpha -isophoron |
| alpha -isophorone |
| ISOACETOPHORON |
| nchem.180-comp3 |
| IPH (CHRIS Code) |
| ISOPHORONE [MI] |
| Isophorone Reagent Grade |
| ISOPHORONE [FHFI] |
| ISOPHORONE [HSDB] |
| SCHEMBL22522 |
| Isophorone, >=97%, FG |
| BIDD:ER0627 |
| Isophorone, analytical standard |
| CHEMBL1882894 |
| FEMA 3553 |
| 3,5,5-Trimethylcyclohex-2-enon |
| 3,5,5-trimethyl-cyclohex-2-enone |
| HY-Y0932 |
| NSC-4881 |
| Tox21_202312 |
| Tox21_300050 |
| BBL027346 |
| MFCD00001584 |
| NSC403657 |
| s2998 |
| STK801792 |
| AKOS000120392 |
| 3,5,5-trimethylcyclohex-2-ene-1-one |
| 3,5,5-trimethylcyclohexa-2-en-1-one |
| LS-1750 |
| 3,3,5-trimethyl-cyclohex-5-en-1-one |
| 3,5,5-Trimethyl-2-cyclo-hexen-1-one |
| 3,5,5-trimethyl-cyclohex-2-en-1-one |
| 1,1, 3-Trimethyl-3-cyclohexene-5-one |
| 1,3,3-TRIMETHYLCYLOHEXEN-5-ONE |
| 2-ciclohexen-1-ona, 3,5,5-trimetil- |
| 3,5, 5-Trimethyl-2-cyclohexene-1-one |
| NCGC00164006-01 |
| NCGC00164006-02 |
| NCGC00164006-03 |
| NCGC00254115-01 |
| NCGC00259861-01 |
| 3,3,5-trimethyl-cyclohex-5 -en-1-one |
| AC-10580 |
| VS-08530 |
| 1,5,5-TRIMETHYL-3-OXOCYCLOHEXENE |
| 3,5,5 - trimethylcyclohex - 2 - enone |
| Isophorone, Vetec(TM) reagent grade, 97% |
| CS-0015924 |
| FT-0627443 |
| I0151 |
| EN300-20384 |
| D72515 |
| A839454 |
| Q415519 |
| W-104274 |
| F0001-2053 |
| Z104477948 |
| InChI=1/C9H14O/c1-7-4-8(10)6-9(2,3)5-7/h4H,5-6H2,1-3H |
| 2-CYCLOHEXEN-1-ONE, 3,5,5-TRIMETHYL-2-ETHOXY-3,4-DI-HYDRO-2-PYRAN |
|
There are more than 10 synonyms. If you wish to see them all click here.
|