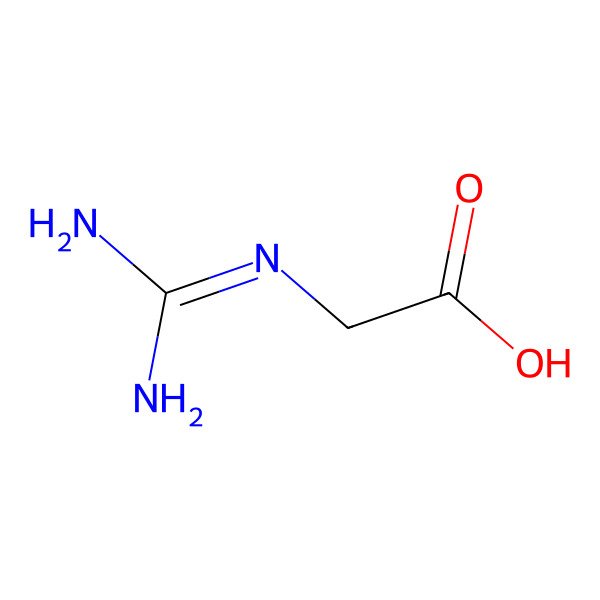
| guanidinoacetic acid |
| 352-97-6 |
| Guanidineacetic acid |
| 2-Guanidinoacetic acid |
| Guanidoacetic acid |
| N-amidinoglycine |
| Betacyamine |
| Guanyl glycine |
| Betasyamine |
| guanidinoacetate |
| Glykocyamin |
| Guanidylacetic acid |
| GLYCINE, N-AMIDINO- |
| Guanidine, (carboxymethyl)- |
| glycine, N-(aminoiminomethyl)- |
| N-(Aminoiminomethyl)glycine |
| N-[AMINO(IMINO)METHYL]GLYCINE |
| 2-carbamimidamidoacetic acid |
| Acetic acid, ((aminoiminomethyl)amino)- |
| amidinoglycine |
| GUANIDINO ACETATE |
| N-(carbamimidoyl)glycine |
| NSC 1901 |
| (carbamimidamido)acetic acid |
| N-carbamimidoylglycine |
| EINECS 206-529-5 |
| Guanidineacetic--d2 Acid |
| MFCD00004278 |
| .alpha.-Guanidinoacetic acid |
| N-(aminoiminomethyl)-glycine |
| UNII-GO52O1A04E |
| AI3-17119 |
| CHEMBL281593 |
| GO52O1A04E |
| CHEBI:16344 |
| NSC1901 |
| NSC-1901 |
| NSC-26360 |
| NSC-227847 |
| n-amidino-glycine |
| NMG |
| N-Guanylglycine |
| Acetic acid, [(aminoiminomethyl)amino]- |
| 2-[[Amino(imino)methyl]amino]acetic acid |
| 2-{[amino(imino)methyl]amino}acetic acid |
| 2-((AMINO(IMINO)METHYL)AMINO)ACETIC ACID |
| Glucocyamine |
| Guanidoacetate |
| Guanidylacetate |
| a-Guanidinoacetate |
| b-Guanidinoacetate |
| beta-Guanidinoacetate |
| guanidine acetic acid |
| alpha-Guanidinoacetate |
| a-Guanidinoacetic acid |
| b-Guanidinoacetic acid |
| beta-Guanidinoacetic acid |
| GUANIDOACETIC_ACID |
| (carboxymethyl)-Guanidine |
| alpha-Guanidinoacetic acid |
| GLYCOCYAMINE [MI] |
| bmse000384 |
| D0AH1H |
| Guanidineacetic acid, 99% |
| SCHEMBL33970 |
| 2-carbamimidamido-acetic acid |
| AMIDINOGLYCINE [INCI] |
| Guanidine acetic acid, 99% |
| GLYCOCYAMINE [WHO-DD] |
| Glycine, N-amidino- (8CI) |
| GTPL5325 |
| (Diaminomethylenamino)-essigsaure |
| DTXSID50861885 |
| [(aminoiminomethyl)amino]-Acetate |
| HMS3604I10 |
| GAA; Glycocyamine; Guanidinoacetate |
| NSC26360 |
| AC2229 |
| BDBM50021601 |
| NSC227847 |
| s6340 |
| STL164350 |
| [(aminoiminomethyl)amino]-Acetic acid |
| 2-[[Amino(imino)methyl]amino]acetate |
| AKOS005363765 |
| DB02751 |
| HY-W021448 |
| 2-[(diaminomethylidene)amino]acetic acid |
| ([Amino(imino)methyl]amino)acetic acid # |
| AS-10760 |
| SY015122 |
| LS-174518 |
| CS-0040590 |
| FT-0626779 |
| FT-0669070 |
| G0167 |
| EN300-49151 |
| C00581 |
| Q2740979 |
| W-106678 |
| Z586248684 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|