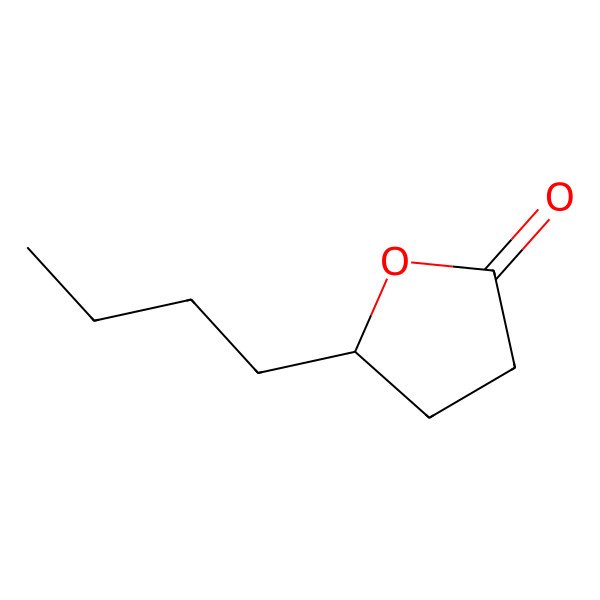
| 104-50-7 |
| gamma-Octanoic lactone |
| 5-Butyldihydrofuran-2(3H)-one |
| 4-Octanolide |
| 5-butyloxolan-2-one |
| 2(3H)-Furanone, 5-butyldihydro- |
| Octan-4-olide |
| 4-Hydroxyoctanoic acid lactone |
| Octanolide-1,4 |
| 8-Oxo-5-octanolide |
| gamma-Octanolactone |
| 5-Butyldihydro-2(3H)-furanone |
| 5-Butyltetrahydro-2-furanone |
| 4-Butyl-gamma-butyrolactone |
| Octanoic acid, gamma lactone |
| gamma-Butyl-gamma-butyrolactone |
| gamma-N-Butyl-gamma-butyrolactone |
| .gamma.-Octalactone |
| Octano-1,4-lactone |
| 2(3H)-Furanone, dihydro-5-butyl- |
| FEMA No. 2796 |
| gamma-octanolide |
| Gamma Octalactone |
| gamma-Octalactone (natural) |
| NSC 24270 |
| 4-Butyl-4-hydroxybutyric acid lactone |
| .gamma.-Octanolactone |
| Octanoic acid, 4-hydroxy-, lactone |
| .gamma.-Butyl-.gamma.-butyrolactone |
| EINECS 203-208-1 |
| .gamma.-Butylbutyrolactone |
| UNII-UHD6M52X0K |
| BRN 0111677 |
| UHD6M52X0K |
| 4-Butyl-.gamma.-butyrolactone |
| AI3-35896 |
| Octanoic acid, .gamma. lactone |
| DTXSID1047609 |
| 5-Butyl-dihydrofuran-2(3H)-one |
| MFCD00005402 |
| NSC-24270 |
| NSC-26509 |
| .gamma.-N-Butyl-.gamma.-butyrolactone |
| 4-Hydroxyoctanoic acid, .gamma.-lactone |
| Octanoic acid, 4-hydroxy-, .gamma.-lactone |
| WLN: T5OVTJ E4 |
| 5-17-09-00073 (Beilstein Handbook Reference) |
| Octanoic acid, .gamma.-lactone |
| (r)-4-octanolide |
| -Octanolactone |
| octan-4-olid |
| Nat.Gamma-Octalactone |
| .gamma. -0ctalactone |
| gamma-Butylbutyrolactone |
| octan - 4 - olide |
| gamma -Octanoic lactone |
| gamma-Octanoic lactone, 97% |
| SCHEMBL111822 |
| 5-Butylhydro-2(3H)-furanone |
| GAMMA-OCTALACTONE [FCC] |
| 5-butyl-tetrahydro-furan-2-one |
| CHEMBL3559967 |
| DTXCID9027609 |
| 5-Butyl-1-oxacyclopentan-2-one |
| FEMA 2796 |
| IPBFYZQJXZJBFQ-UHFFFAOYSA- |
| (RS)-.GAMMA.-OCTALACTONE |
| 5-butyl dihydro-2(3H)-furanone |
| CHEBI:145738 |
| dihydro-5-butyl-2(3H)-furanone |
| 2(3h)-furanona, 5-butildihidro- |
| .GAMMA.-OCTALACTONE [FHFI] |
| 5-Butyldihydro-2(3H)-furanone # |
| NSC24270 |
| NSC26509 |
| 4-Hydroxyoctanoic acid gamma-lactone |
| Tox21_302569 |
| AC2057 |
| LMFA07040016 |
| NSC 26509 |
| gamma-Octalactone, analytical standard |
| Lactone de l'acide 5-hydroxyoctanoque |
| AKOS015904720 |
| CS-W018278 |
| LS-2993 |
| 2-FLUORO-BENZIMIDICACIDETHYLESTER |
| gamma-Octalactone, >=97%, FCC, FG |
| Octanoic acid 4-hydroxy-lactone (6CI) |
| NCGC00256744-01 |
| BS-14348 |
| CAS-104-50-7 |
| gamma-Octalactone, natural, >=97%, FG |
| SY015123 |
| Octanoic acid, 4-hydroxy-, lactone (6CI) |
| FT-0618727 |
| H0232 |
| EN300-253946 |
| A800991 |
| A866726 |
| W-108811 |
| Q27291078 |
| InChI=1/C8H14O2/c1-2-3-4-7-5-6-8(9)10-7/h7H,2-6H2,1H3 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|