Cycloheximide
| Internal ID | bd6dbe88-ea34-4cb6-8414-b4c1d76f9ef8 |
| Taxonomy | Organoheterocyclic compounds > Pyridines and derivatives > Hydropyridines > Tetrahydropyridines |
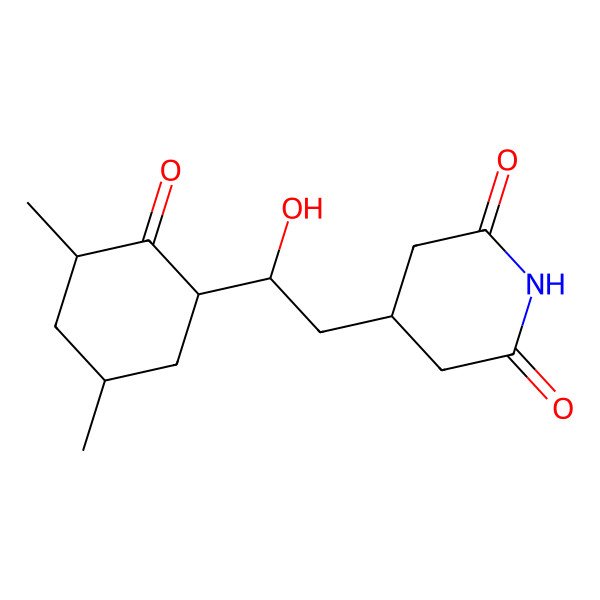
| IUPAC Name | 4-[(2R)-2-[(1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl]-2-hydroxyethyl]piperidine-2,6-dione |
| SMILES (Canonical) | CC1CC(C(=O)C(C1)C(CC2CC(=O)NC(=O)C2)O)C |
| SMILES (Isomeric) | C[C@H]1C[C@@H](C(=O)[C@@H](C1)[C@@H](CC2CC(=O)NC(=O)C2)O)C |
| InChI | InChI=1S/C15H23NO4/c1-8-3-9(2)15(20)11(4-8)12(17)5-10-6-13(18)16-14(19)7-10/h8-12,17H,3-7H2,1-2H3,(H,16,18,19)/t8-,9-,11-,12+/m0/s1 |
| InChI Key | YPHMISFOHDHNIV-FSZOTQKASA-N |
| Popularity | 39,821 references in papers |
| Molecular Formula | C15H23NO4 |
| Molecular Weight | 281.35 g/mol |
| Exact Mass | 281.16270821 g/mol |
| Topological Polar Surface Area (TPSA) | 83.50 Ų |
| XlogP | 0.50 |
| Atomic LogP (AlogP) | 1.04 |
| H-Bond Acceptor | 4 |
| H-Bond Donor | 2 |
| Rotatable Bonds | 3 |
| 66-81-9 |
| ACTIDIONE |
| Cicloheximide |
| NARAMYCIN A |
| Kaken |
| Actidion |
| Actidone |
| Hizarocin |
| Naramycin |
| Neocycloheximide |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| Human Intestinal Absorption | + | 0.9371 | 93.71% |
| Caco-2 | - | 0.5983 | 59.83% |
| Blood Brain Barrier | + | 0.5080 | 50.80% |
| Human oral bioavailability | - | 0.5857 | 58.57% |
| Subcellular localzation | Mitochondria | 0.7938 | 79.38% |
| OATP2B1 inhibitior | - | 0.8485 | 84.85% |
| OATP1B1 inhibitior | + | 0.9413 | 94.13% |
| OATP1B3 inhibitior | + | 0.9480 | 94.80% |
| MATE1 inhibitior | - | 0.9800 | 98.00% |
| OCT2 inhibitior | - | 0.9750 | 97.50% |
| BSEP inhibitior | - | 0.9604 | 96.04% |
| P-glycoprotein inhibitior | - | 0.8887 | 88.87% |
| P-glycoprotein substrate | + | 0.5000 | 50.00% |
| CYP3A4 substrate | - | 0.5079 | 50.79% |
| CYP2C9 substrate | - | 0.5969 | 59.69% |
| CYP2D6 substrate | - | 0.8308 | 83.08% |
| CYP3A4 inhibition | - | 0.9133 | 91.33% |
| CYP2C9 inhibition | - | 0.9098 | 90.98% |
| CYP2C19 inhibition | - | 0.9382 | 93.82% |
| CYP2D6 inhibition | - | 0.9230 | 92.30% |
| CYP1A2 inhibition | - | 0.9529 | 95.29% |
| CYP2C8 inhibition | - | 0.9353 | 93.53% |
| CYP inhibitory promiscuity | - | 0.9635 | 96.35% |
| UGT catelyzed | + | 0.9000 | 90.00% |
| Carcinogenicity (binary) | - | 0.8313 | 83.13% |
| Carcinogenicity (trinary) | Non-required | 0.6136 | 61.36% |
| Eye corrosion | - | 0.9904 | 99.04% |
| Eye irritation | - | 0.9294 | 92.94% |
| Skin irritation | + | 0.5000 | 50.00% |
| Skin corrosion | - | 0.9463 | 94.63% |
| Ames mutagenesis | - | 0.9500 | 95.00% |
| Human Ether-a-go-go-Related Gene inhibition | - | 0.5383 | 53.83% |
| Micronuclear | + | 0.7800 | 78.00% |
| Hepatotoxicity | + | 0.5927 | 59.27% |
| skin sensitisation | - | 0.9370 | 93.70% |
| Respiratory toxicity | + | 0.6556 | 65.56% |
| Reproductive toxicity | + | 0.9222 | 92.22% |
| Mitochondrial toxicity | + | 0.7000 | 70.00% |
| Nephrotoxicity | + | 0.4619 | 46.19% |
| Acute Oral Toxicity (c) | I | 0.8338 | 83.38% |
| Estrogen receptor binding | + | 0.8905 | 89.05% |
| Androgen receptor binding | + | 0.8697 | 86.97% |
| Thyroid receptor binding | + | 0.8461 | 84.61% |
| Glucocorticoid receptor binding | - | 0.4932 | 49.32% |
| Aromatase binding | - | 0.7712 | 77.12% |
| PPAR gamma | + | 0.8611 | 86.11% |
| Honey bee toxicity | - | 0.7492 | 74.92% |
| Biodegradation | - | 0.7000 | 70.00% |
| Crustacea aquatic toxicity | - | 0.6700 | 67.00% |
| Fish aquatic toxicity | - | 0.5122 | 51.22% |
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL3577 | P00352 | Aldehyde dehydrogenase 1A1 |
31622.8 nM |
Potency |
via CMAUP
|
| CHEMBL4096 | P04637 | Cellular tumor antigen p53 |
251.2 nM 251.2 nM |
Potency Potency |
via CMAUP
via Super-PRED |
| CHEMBL340 | P08684 | Cytochrome P450 3A4 |
15848.9 nM 15848.9 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL2052028 | P60842 | Eukaryotic initiation factor 4A-I |
87 nM |
IC50 |
via Super-PRED
|
| CHEMBL1902 | P62942 | FK506-binding protein 1A |
3600 nM |
IC50 |
PMID: 10479292
|
| CHEMBL2535 | P11166 | Glucose transporter |
228 nM |
IC50 |
DOI: 10.6019/CHEMBL3433997
|
| CHEMBL3880 | P07900 | Heat shock protein HSP 90-alpha |
<
195 nM |
AC50 |
via CMAUP
|
| CHEMBL5514 | P42858 | Huntingtin |
354.8 nM |
Potency |
via Super-PRED
|
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha |
63.1 nM |
Potency |
via Super-PRED
|
| CHEMBL4040 | P28482 | MAP kinase ERK2 |
39.8 nM |
Potency |
via Super-PRED
|
| CHEMBL1293277 | O15118 | Niemann-Pick C1 protein |
446.7 nM 316.2 nM 446.7 nM |
Potency Potency Potency |
via Super-PRED
via Super-PRED via CMAUP |
| CHEMBL1293294 | P51151 | Ras-related protein Rab-9A |
316.2 nM 316.2 nM 316.2 nM |
Potency Potency Potency |
via CMAUP
via Super-PRED via Super-PRED |
| CHEMBL1293232 | Q16637 | Survival motor neuron protein |
1778.3 nM 446.7 nM 2818.4 nM 891.3 nM |
Potency Potency Potency Potency |
via CMAUP
via Super-PRED via CMAUP via Super-PRED |
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL253 | P34972 | Cannabinoid CB2 receptor | 98.34% | 97.25% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 96.96% | 96.09% |
| CHEMBL2581 | P07339 | Cathepsin D | 96.17% | 98.95% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 89.78% | 91.11% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 89.52% | 97.09% |
| CHEMBL299 | P17252 | Protein kinase C alpha | 86.65% | 98.03% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 86.32% | 95.56% |
| CHEMBL218 | P21554 | Cannabinoid CB1 receptor | 85.11% | 96.61% |
| CHEMBL1293267 | Q9HC97 | G-protein coupled receptor 35 | 84.30% | 89.34% |
| CHEMBL5103 | Q969S8 | Histone deacetylase 10 | 83.53% | 90.08% |
| CHEMBL1994 | P08235 | Mineralocorticoid receptor | 82.37% | 100.00% |
| CHEMBL213 | P08588 | Beta-1 adrenergic receptor | 81.47% | 95.56% |
| CHEMBL2553 | Q15418 | Ribosomal protein S6 kinase alpha 1 | 81.12% | 85.11% |
| CHEMBL3045 | P05771 | Protein kinase C beta | 80.99% | 97.63% |
| CHEMBL255 | P29275 | Adenosine A2b receptor | 80.32% | 98.59% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| PubChem | 6197 |
| NPASS | NPC253703 |
| ChEMBL | CHEMBL123292 |
| LOTUS | LTS0227054 |
| wikiData | Q412895 |