| 58864-81-6 |
| D-alpha-Tocotrienol |
| zeta1-Tocopherol |
| (R)-alpha-Tocotrienol |
| D-|A-Tocotrienol |
| (2R,3'E,7'E)-alpha-Tocotrienol |
| Tocotrienol, alpha |
| 1721-51-3 |
| alpha-Tocotrienol [MI] |
| UNII-B6LXL1832Y |
| B6LXL1832Y |
| CHEBI:33270 |
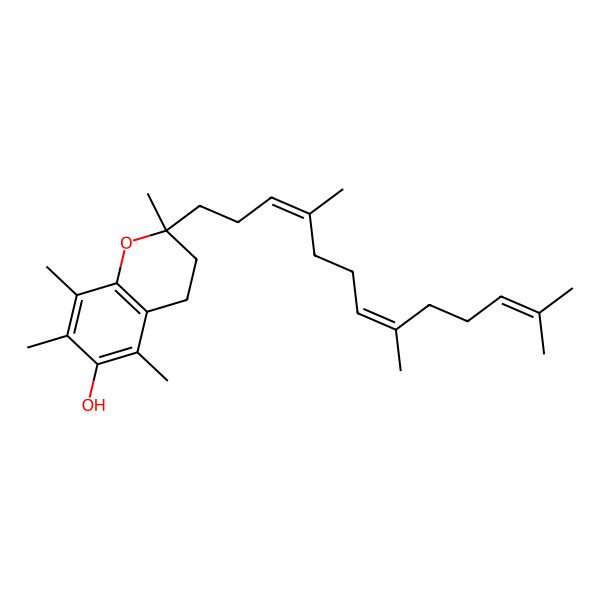
| (2R)-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl]chroman-6-ol |
| (2R)-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl]-3,4-dihydrochromen-6-ol |
| (2R)-2,5,7,8-Tetramethyl-2-((3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl)chroman-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-((3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl)-, (2R)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-, (R-(E,E))- |
| dl-alpha-Tocotrienol |
| 2R,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl]-3,4-dihydro-2H-chromen-6-ol |
| 5,7,8-Trimethyltocotrienol |
| alpha -Tocotrienol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-, [R-(E,E)]- |
| 2H-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-, (2R)- |
| .alpha.-Tocotrienol |
| delta-alpha-tocotrienol |
| D-ALPHA TOCOTRIENOL |
| D-.ALPHA.-TOCOTRIENOL |
| SCHEMBL242672 |
| CHEMBL120276 |
| Tocotrienol, 5,7,8-trimethyl |
| SCHEMBL16430461 |
| (R)-.ALPHA.-TOCOTRIENOL |
| .ALPHA.-TOCOTRIENOL [MI] |
| DTXSID901019976 |
| HMS3650C04 |
| ALPHA-TOCOTRIENOL [WHO-DD] |
| LMPR02020054 |
| MFCD11045308 |
| alpha-Tocotrienol, analytical standard |
| VITAMIN E (ALPHA-TOCOTRIENOL) |
| NCGC00253538-02 |
| AS-78670 |
| HY-129459 |
| CS-0105636 |
| (2R,3'E,7'E)-.ALPHA.-TOCOTRIENOL |
| D-alpha-Tocotrienol, analytical reference material |
| Q171833 |
| SR-01000946263 |
| J-010798 |
| SR-01000946263-1 |
| 2,5,7,8-tetramethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-6-chromanol |
| (2R)-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl]-3,4-dihydro-2H-chromen-6-ol |
| (2R)-2beta,5,7,8-Tetramethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-3,4-dihydro-2H-1-benzopyran-6-ol |
| (2R)-3,4-Dihydro-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-2H-1-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-, (2R)- (9CI) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|