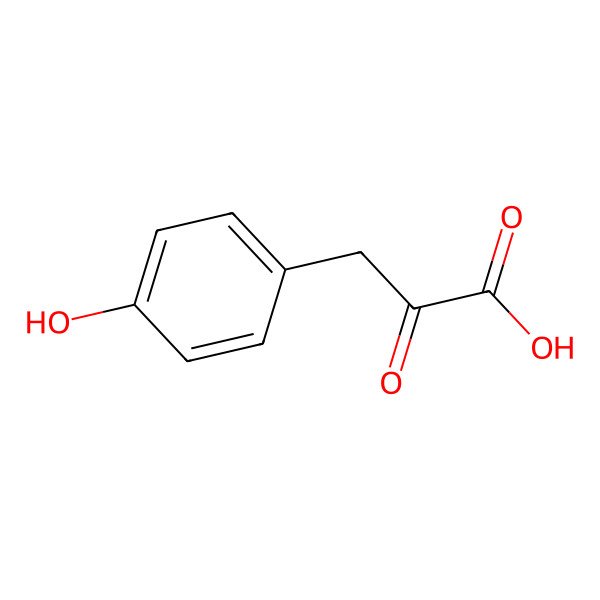
| 156-39-8 |
| 3-(4-Hydroxyphenyl)-2-oxopropanoic acid |
| Testacid |
| p-Hydroxyphenylpyruvic acid |
| 4-hydroxyphenylpyruvate |
| 3-(4-Hydroxyphenyl)pyruvic acid |
| Testacide |
| (P-HYDROXYPHENYL)PYRUVIC ACID |
| 3-(p-Hydroxyphenyl)pyruvic acid |
| (4-hydroxyphenyl)pyruvic acid |
| p-hydroxyphenylpyruvate |
| 3-(p-Hydroxyphenyl)-2-oxopropanoic acid |
| Pyruvic acid, (p-hydroxyphenyl)- |
| 3-(4-HYDROXY-PHENYL)PYRUVIC ACID |
| HPPA |
| Pyruvic acid, p-hydroxyphenyl- |
| NSC 100738 |
| 4-Hydroxy alpha-oxobenzenepropanoic acid |
| 3-(4-Hydroxyphenyl)pyruvate |
| Benzenepropanoic acid, 4-hydroxy-.alpha.-oxo- |
| BRN 2691632 |
| Benzenepropanoic acid, 4-hydroxy-alpha-oxo- |
| 4-Hydroxy-a-oxobenzenepropanoic acid |
| EINECS 205-852-9 |
| 4-?Hydroxyphenylpyruvic acid |
| UNII-0YP1694WNQ |
| 3-(p-Hydroxyphenyl)-2-oxopropionic acid |
| 3-(4-Hydroxyphenyl)-2-oxopropionic acid |
| 3-(4-hydroxyphenyl)-2-oxo-propanoic acid |
| 0YP1694WNQ |
| CHEBI:15999 |
| MFCD00002591 |
| NSC666757 |
| NSC-100738 |
| NSC-666757 |
| 4-Hydroxy-alpha-oxobenzenepropanoic acid |
| hydroxyphenylpyruvate |
| 4-10-00-03630 (Beilstein Handbook Reference) |
| Benzenepropanoic acid, 4-hydroxy-alpha-oxo- (9CI) |
| (p-hydroxyphenyl)-pyruvic acid |
| 3-(p-Hydroxyphenyl)pyruvate |
| ENO |
| p-hydroxyphenylpyruvic |
| 4HPPA |
| 4-Hydroxy-Phenylpyruvate |
| (p-Hydroxyphenyl)pyruvate |
| 4-ydroxyphenylpyruvic acid |
| WLN: QVV1R DQ |
| (p-hydroxyphenyl)-Pyruvate |
| 4-HPPA |
| bmse000262 |
| D05VRD |
| D0G6RI |
| 4- Hydroxyphenylpyruvic acid |
| SCHEMBL24157 |
| CHEMBL607712 |
| GTPL6629 |
| 4-Hydroxy-a-oxobenzenepropanoate |
| AMY4735 |
| DTXSID80166017 |
| 4-Hydroxyphenylpyruvic acid, 98% |
| STR05283 |
| 4-Hydroxy-alpha-oxobenzenepropanoate |
| 3-(p-Hydroxyphenyl)-2-oxopropionate |
| 4-HPPA, p-Hydroxyphenylpyruvic acid |
| ARJ102978 |
| BBL104224 |
| GEO-01551 |
| NSC100738 |
| s2995 |
| STL558218 |
| 3-(4-Hydroxyphenyl)-2-oxopropionate |
| AKOS000136920 |
| 3-(4-hydroxyphenyl)-2-oxo-propanoate |
| AC-7498 |
| CS-W010756 |
| DB07718 |
| FD10465 |
| HY-W010040 |
| NSC 666757 |
| 3-(4-Hydroxyphenyl)-2-oxopropanoicacid |
| Benzenepropanoic acid, ?-hydroxy-?-oxo- |
| p-Hydroxyphenyl-brenztraubensA currencyure |
| PD005526 |
| Pyruvic acid, (p-hydroxyphenyl)- (8CI) |
| 3-(4-hydroxyphenyl)-2-oxo-propionic acid |
| LS-139796 |
| 3-(4-hydroxy phenyl)-2-oxo propanoic acid |
| 3-(4-Hydroxyphenyl)-2-oxopropanoic acid # |
| FT-0618738 |
| C01179 |
| H-7200 |
| EN300-1855716 |
| 4-HYDROXY-.ALPHA.-OXOBENZENEPROPANOIC ACID |
| A809744 |
| Q2823224 |
| 87E988C4-F26C-4E03-9A40-1B0C0B36CA67 |
| 4-Hydroxyphenylpyruvic acid, Grade II, 98-100%, crystalline |
|
There are more than 10 synonyms. If you wish to see them all click here.
|