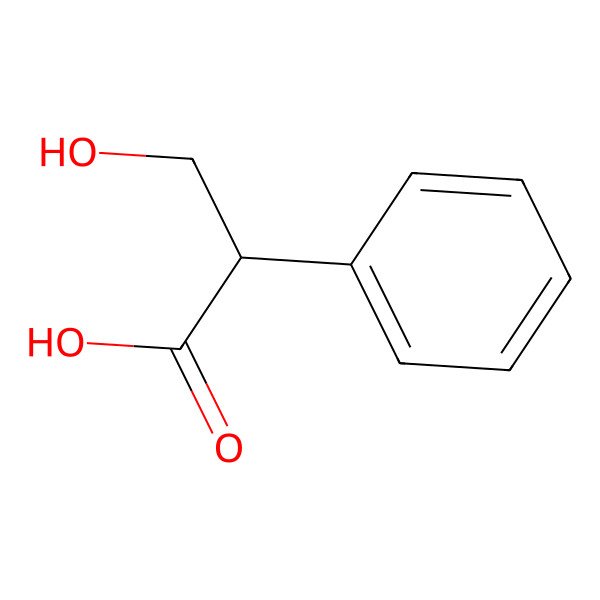
| dl-Tropic acid |
| 552-63-6 |
| 3-hydroxy-2-phenylpropanoic acid |
| 529-64-6 |
| 2-Phenylhydracrylic acid |
| 3-Hydroxy-2-phenylpropionic acid |
| Tropate |
| alpha-(Hydroxymethyl)benzeneacetic acid |
| (+-)-tropic acid |
| Hydracrylic acid, 2-phenyl- |
| (+/-)-Tropic acid |
| beta-hydroxyhydratropic acid |
| MFCD00004255 |
| alpha-(Hydroxymethyl)phenylacetic acid |
| Hydratropic acid, beta-hydroxy- |
| alpha-Phenyl-beta-hydroxypropionic acid |
| alpha-Toluic acid, alpha-(hydroxymethyl)- |
| 28845-94-5 |
| UNII-9RM4U80765 |
| Benzeneacetic acid, .alpha.-(hydroxymethyl)- |
| Hydratropic acid, .beta.-hydroxy- |
| EINECS 208-465-3 |
| EINECS 209-020-6 |
| (1)-(Hydroxymethyl)phenylacetic acid |
| NSC 20990 |
| NSC-20990 |
| 9RM4U80765 |
| (+/-)-2-Phenyl-3-hydroxypropionic acid |
| Benzeneacetic acid, alpha-(hydroxymethyl)- |
| 2-phenylhydracrylate |
| (2RS)-3-Hydroxy-2-phenylpropanoic Acid (Tropic Acid) |
| tropasaure |
| tropaic acid |
| di-Tropic acid |
| MFCD00211262 |
| 3-Hydroxy-2-phenylpropanoicacid |
| 3-hydroxy-2-phenyl-propanoic acid |
| Racemic tropic acid |
| b-Hydroxyhydratropate |
| Tropic acid, 98% |
| DL-TROPICACID |
| (+-)-Tropate |
| Tropicamide impurity C |
| beta-Hydroxyhydratropate |
| b-Hydroxyhydratropic acid |
| TROPIC ACID [MI] |
| Tropic acid, (R)-isomer |
| Tropic acid, (S)-isomer |
| bmse000413 |
| alpha-Phenylhydracrylic acid |
| Cambridge id 5132935 |
| a-Phenyl-b-hydroxypropionate |
| Tropic acid, (+-)-isomer |
| Oprea1_210445 |
| Tropic acid, monosodium salt |
| 3-Hydroxy-2-phenylpropionate |
| a-(Hydroxymethyl)phenylacetate |
| SCHEMBL468727 |
| a-(Hydroxymethyl)benzeneacetate |
| Tropic acid, (.+/-.)- |
| a-Phenyl-b-hydroxypropionic acid |
| CHEBI:30765 |
| 2-phenyl-3-hydroxypropanoic acid |
| DTXSID90862179 |
| a-(hydroxymethyl)phenylacetic acid |
| alpha-(Hydroxymethyl)phenylacetate |
| 3-hydroxy-2-phenyl-propionic acid |
| alpha-(Hydroxymethyl)benzeneacetate |
| alpha-Phenyl-beta-hydroxypropionate |
| DL-TROPIC ACID;TROPIC ACID |
| HMS1577B17 |
| TROPIC ACID, (+/-)- |
| 2-hydroxymethyl-2-phenylacetic acid |
| AMY40428 |
| NSC20990 |
| BBL010661 |
| s5689 |
| STK801680 |
| (+-)-(hydroxymethyl)phenylacetic acid |
| AKOS005609216 |
| CS-W021934 |
| HY-W041194 |
| PS-5286 |
| SDCCGMLS-0064629.P001 |
| 2-PHENYL-3-HYDROXYPROPIONIC ACID |
| AC-23896 |
| SY009488 |
| SY030594 |
| FT-0625524 |
| FT-0651762 |
| T0533 |
| Tropic acid, Vetec(TM) reagent grade, 98% |
| TROPICAMIDE IMPURITY C [EP IMPURITY] |
| Tropic acid 3-Hydroxy-2-phenylpropanoic acid |
| .alpha.-Toluic acid, .alpha.-(hydroxymethyl)- |
| C01456 |
| EN300-105884 |
| (+/-)-3-HYDROXY-2-PHENYLPROPIONIC ACID |
| (2RS)-3-HYDROXY-2-PHENYLPROPANOIC ACID |
| ATROPINE SULFATE IMPURITY C [EP IMPURITY] |
| Q2823318 |
| W-105782 |
| Z1080722424 |
| 9392A742-913E-4F50-B2BE-B3E01B793DD6 |
| Benzeneacetic acid, .alpha.-(hydroxymethyl)-, (.+/-.)- |
| BENZENEACETIC ACID, .ALPHA.-(HYDROXYMETHYL)-, (+/-)- |
| Tropicamide impurity C, European Pharmacopoeia (EP) Reference Standard |
|
There are more than 10 synonyms. If you wish to see them all click here.
|