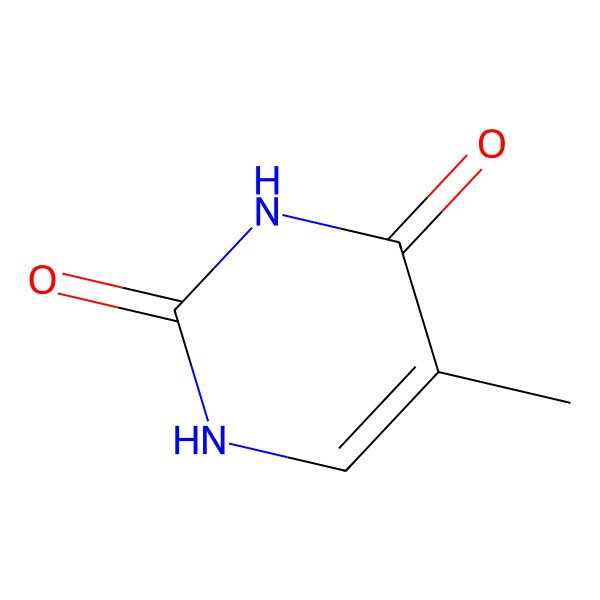
| 5-methyluracil |
| 65-71-4 |
| 2,4-Dihydroxy-5-methylpyrimidine |
| Thymin |
| 5-methylpyrimidine-2,4(1H,3H)-dione |
| Thymine anhydrate |
| 2,4(1H,3H)-Pyrimidinedione, 5-methyl- |
| Thymin (purine base) |
| 5-methyl-2,4(1H,3H)-pyrimidinedione |
| 5-Methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione |
| 5-methyl-1H-pyrimidine-2,4-dione |
| 5-Methyl Uracil |
| CCRIS 5584 |
| CHEBI:17821 |
| Thymine-t |
| UNII-QR26YLT7LT |
| QR26YLT7LT |
| AI3-25479 |
| NSC14705 |
| EINECS 200-616-1 |
| NSC 14705 |
| 5-methylpyrimidine-2,4-diol |
| Thymine (VAN) |
| 5-Methylpyrimidine-2,4-dione |
| (3H)Methylthymidine |
| Methyl-3H thymidine |
| Thymine (8CI) |
| 4-Hydroxy-5-methylpyrimidin-2(1H)-one |
| Zidovudine Impurity C |
| DTXSID4052342 |
| Thymine-methyl-d3-6-d |
| MFCD00006026 |
| NSC-14705 |
| CHEMBL993 |
| 5-METHYLPYRIMIDINE-2,4(1H,3H)-DIONE (THYMINE) |
| THYMINE-13C1 |
| DTXCID4030914 |
| 5-Methyl-2,4-dihydroxypyrimidine |
| NSC-168663 |
| 123430-67-1 |
| 153445-43-3 |
| Thy |
| 3059-73-2 |
| THYMINE (USP IMPURITY) |
| THYMINE [USP IMPURITY] |
| 2,4(1H,3H)-Pyrimidinedione, 5-methyl-, labeled with tritium |
| 5 Methyluracil |
| 2792-47-4 |
| ZIDOVUDINE IMPURITY C (EP IMPURITY) |
| ZIDOVUDINE IMPURITY C [EP IMPURITY] |
| 5-methy uracil(Thymine) |
| 5-methyl-uracil |
| Thymine,(S) |
| THYMINE [INCI] |
| Thymine, >=99% |
| THYMINE [MI] |
| Zidovudine EP Impurity C |
| THYMINE [WHO-DD] |
| STAVUINE IMPURITY A |
| D0F4KL |
| Epitope ID:167476 |
| SCHEMBL5235 |
| 2,4(1H,3H)-Pyrimidinedione, 5-methyl- (9CI) |
| 4(1H)-Pyrimidinone, 2-hydroxy-5-methyl- (9CI) |
| 4(3H)-Pyrimidinone, 2-hydroxy-5-methyl- (9CI) |
| 5-Methyl-2,4-dioxypyrimidine |
| 5-Methylpyrimidine-2 4-dione |
| 2.6-Dioxy-5-methyl-pyrimidin |
| GTPL4581 |
| SCHEMBL15496760 |
| SCHEMBL16356870 |
| 24-Dihydroxy-5-methylpyrimidine |
| 5-Methyl-2 4-dihydroxypyrimidine |
| WLN: T6N CNJ BQ DQ E1 |
| BCP22973 |
| 2,3H)-Pyrimidinedione, 5-methyl- |
| Tox21_303929 |
| BDBM50134397 |
| s9382 |
| STL280241 |
| STL477641 |
| STAVUINE IMPURITY A [WHO-IP] |
| AKOS000120923 |
| AKOS002337369 |
| AC-7756 |
| AM81332 |
| CCG-266101 |
| CS-O-10836 |
| CS-W011166 |
| DB03462 |
| HY-W010450 |
| NSC 168663 |
| SB57778 |
| ZIDOVUDINE IMPURITY C [WHO-IP] |
| 5-Methyl-2 4(1H 3H)-pyrimidinedione |
| CAS-65-71-4 |
| CID 5274265 |
| Timina,2,4-dihidroxi-5-metilpiramidina |
| 4-hidroxi-5-metilpirimidin-2 (1H)-ona |
| NCGC00357169-01 |
| SY014896 |
| Thymine, Vetec(TM) reagent grade, 99% |
| 5-Metil-2, 4 (1H, 3H)-pirimidinediona |
| LS-153797 |
| 2,4 (1h, 3h)-pirimidinadiona, 5-metil- |
| FT-0602550 |
| FT-0675207 |
| FT-0771534 |
| T0234 |
| EN300-21969 |
| C00178 |
| T-3845 |
| Thymine, suitable for cell culture, BioReagent |
| 5-metil-1,2,3,4-tetrahidropirimidina-2,4-diona |
| A835203 |
| Q171973 |
| SR-01000945223 |
| 5-Methyl-1 2 3 4-tetrahydropyrimidine-2 4-dione |
| SR-01000945223-1 |
| 036B9F1D-9B61-4CED-967C-BF1DA180E5C2 |
| F0001-1753 |
| Z147641104 |
| InChI=1/C5H6N2O2/c1-3-2-6-5(9)7-4(3)8/h2H,1H3,(H2,6,7,8,9 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|