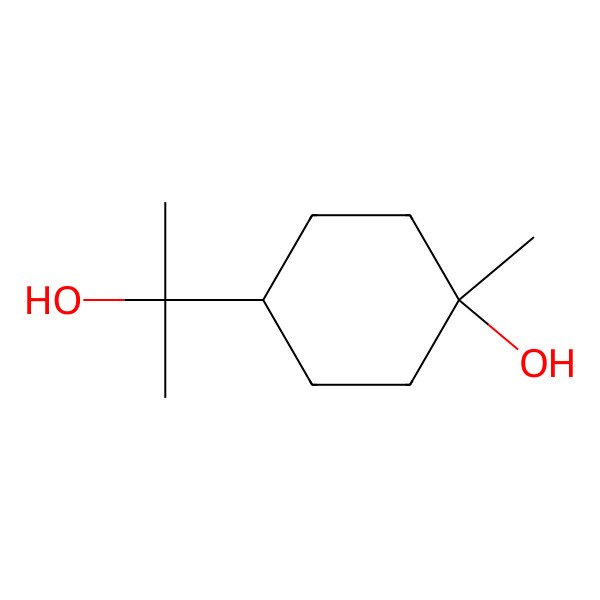
| trans-Terpin |
| p-Menthane-1,8-diol |
| 80-53-5 |
| 565-48-0 |
| cis-p-Menthan-1,8-diol |
| 1,8-Terpin |
| 4-p-Menthan-1,8-diol |
| 565-50-4 |
| 4-(2-hydroxypropan-2-yl)-1-methylcyclohexan-1-ol |
| Terpin, trans- |
| cis-1,8-p-Menthanediol |
| trans-1,8-Terpin |
| trans-p-Menthan-1,8-diol |
| Dipenteneglycol |
| Terpene |
| NSC 403856 |
| Terpin trans-form [MI] |
| trans-1,8-p-Menthanediol |
| trans-p-Menthane-1,8-diol |
| cis-4-Hydroxy-alpha,alpha,4-trimethylcyclohexanemethanol |
| Terpinol |
| Terpin (VAN) |
| Terpin [BAN] |
| NSC-403856 |
| UNII-4HW1S44T5G |
| UNII-MPF495B08R |
| 4HW1S44T5G |
| MPF495B08R |
| Cyclohexanemethanol, 4-hydroxy-.alpha.,.alpha.,4-trimethyl- |
| DTXSID7023643 |
| 4-(2-Hydroxypropan-2-yl)-1-methylcyclohexanol |
| 1,8-Terpenediol |
| EINECS 201-288-2 |
| EINECS 209-279-5 |
| 1,4-Terpin |
| NCGC00159414-02 |
| EC 201-288-2 |
| 4-Hydroxy-alpha,alpha,4-trimethylcyclohexanemethanol |
| Cyclohexanemethanol, 4-hydroxy-alpha,alpha,4-trimethyl- |
| Cyclohexanemethanol, 4-hydroxy-alpha,alpha,4-trimethyl-, trans- |
| Terpinhydrat |
| trans_terpin |
| cis-Terpin |
| Terpin anhydrous |
| p-Mentha-1,8-diol |
| p-menthan-1,8-diol |
| TERPIN [WHO-DD] |
| TERPIN [MI] |
| cis-p-Menthane-1,8-diol |
| 4-(2-Hydroxy-2-propanyl)-1-methylcyclohexanol |
| Cyclohexanemethanol, 4-hydroxy-?,?,4-trimethyl- |
| SCHEMBL19192 |
| DTXCID903643 |
| SCHEMBL5377815 |
| SCHEMBL9536260 |
| CHEMBL1414114 |
| CHEMBL1513871 |
| CHEMBL1651998 |
| CHEBI:134806 |
| CHEBI:179539 |
| DTXSID401031800 |
| DTXSID501031803 |
| Pharmakon1600-01506188 |
| HY-N4324 |
| Tox21_111646 |
| BBL034665 |
| NSC403856 |
| NSC760418 |
| STL356461 |
| AKOS022099020 |
| AKOS024348939 |
| AKOS037514988 |
| CAS-80-53-5 |
| Cyclohexanemethanol,.alpha.,4-trimethyl- |
| NCGC00159414-01 |
| NCGC00159414-03 |
| NCGC00159414-04 |
| NCGC00159414-05 |
| NCGC00159414-06 |
| NCGC00166136-01 |
| AS-83272 |
| NCI60_003817 |
| PD132153 |
| trans-Terpin, analytical standard, for GC |
| CS-0032749 |
| T2344 |
| AB01563167_01 |
| 4-(1-Hydroxy-1-methylethyl)-1-methylcyclohexanol |
| cis-4-Hydroxy-|A,|A,4-trimethylcyclohexanemethanol |
| TERPIN MONOHYDRATE IMPURITY D [EP IMPURITY] |
| W-107311 |
| 4-(1-Hydroxy-1-methylethyl)-1-methylcyclohexanol # |
| Q27259605 |
| Q27284154 |
| (1R,4R)-4-(2-HYDROXYPROPAN-2-YL)-1-METHYLCYCLOHEXAN-1-OL |
| CYCLOHEXANEMETHANOL, 4-HYDROXY-.ALPHA.,.ALPHA.,4-TRIMETHYL-, CIS- |
| CYCLOHEXANEMETHANOL, 4-HYDROXY-.ALPHA.,.ALPHA.,4-TRIMETHYL-, TRANS- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|