| 515-03-7 |
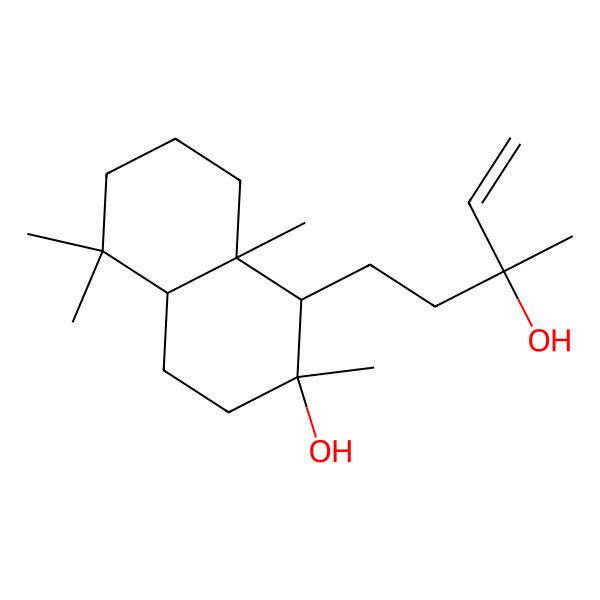
| labd-14-ene-8,13-diol |
| (13R)-Labd-14-ene-8,13-diol |
| Sclareol (natural) |
| SCAREOL |
| (1R,2R,4aS,8aS)-1-((R)-3-hydroxy-3-methylpent-4-en-1-yl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol |
| UNII-B607NP0Q8Y |
| B607NP0Q8Y |
| CHEBI:9053 |
| DTXSID0047111 |
| EINECS 208-194-0 |
| (-)-SCLAREOL |
| BRN 2054148 |
| DTXCID8027111 |
| FEMA NO. 4502 |
| 4-06-00-05554 (Beilstein Handbook Reference) |
| (13R)-Labd-14-ene-8alpha,13-diol |
| Labd-14-ene-8,13-diol, (13R)- |
| (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol |
| (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol |
| Sclareol (6CI) |
| (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol labd-14-ene-8,13-diol |
| 1-Naphthalenepropanol, .alpha.-ethenyldecahydro-2-hydroxy-.alpha.,2,5,5,8a-pentamethyl-, (.alpha.R,1R,2R,4aS,8aS)- |
| 1-Naphthalenepropanol, alpha-ethenyldecahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (1theta-(1alpha(theta),2beta,4abeta,8aalpha))- |
| 1-Naphthalenepropanol, decahydro-alpha-ethenyl-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (1R-(1-alpha(R*),2-beta,4a-beta,8a-alpha))- |
| (1R,2R,4aS,8aS)-1-((3R)-3-hydroxy-3-methylpent-4-en-1-yl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol |
| (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol |
| labd-14-ene-8alpha, 13beta-diol |
| Sclareol, 98% |
| SCLAREOL [INCI] |
| Sclareol, analytical standard |
| SCHEMBL873931 |
| CHEMBL294740 |
| XVULBTBTFGYVRC-HHUCQEJWSA-N |
| (1R - (1a(R*),2b,4ab,8aa)) - 2 - hydroxy - a,2,5,5,8a - pentamethyl - a - vinyldecahydronaphthalene - 1 - propan - 1 - ol |
| (1R-(1alpha(R*),2beta,4Abeta,8aalpha))-2-hydroxy-alpha,2,5,5,8a-pentamethyl-alpha-vinyldecahydronaphthalene-1-propan-1-ol |
| [1R-[1alpha(R*),2beta,4abeta,8aalpha]]-2-hydroxy-alpha,2,5,5,8a-pentamethyl-alpha-vinyldecahydronaphthalene-1-propan-1-ol |
| 1-NAPHTHALENEPROPANOL, .ALPHA.-ETHENYLDECAHYDRO-2-HYDROXY-.ALPHA.,2,5,5,8A-PENTAMETHYL-, (1R-(1.ALPHA.(R*),2.BETA.,4A.BETA.,8A.ALPHA.))- |
| 1-Naphthalenepropanol, .alpha.-ethenyldecahydro-2-hydroxy-.alpha.,2,5,5,8a-pentamethyl-, [1R-[1.alpha.(R*),2.beta.,4a.beta.,8a.alpha.]]- |
| 1-Naphthalenepropanol, alpha-ethenyldecahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (alphaR,1R,2R,4aS,8aS)- |
| HY-N0128 |
| Tox21_302727 |
| MFCD00869558 |
| Sclareol 1000 microg/mL in Acetone |
| AKOS025310185 |
| LMPR0104030010 |
| (1R,2R,8aS)-Decahydro-1-(3-hydroxy-3-methyl-4-pentenyl)-2,5,5,8a-tetramethyl-2-naphthol |
| NCGC00256908-01 |
| AC-34890 |
| AS-14857 |
| CAS-515-03-7 |
| CS-0007834 |
| S0916 |
| C09183 |
| A828631 |
| Q63396017 |
| (1R,2R,4As,8aR)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol |
| (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methyl-pent-4-enyl]-2,5,5,8a-tetramethyl-decalin-2-ol |
| (1R,2R,8aS)-1-((R)-3-hydroxy-3-methylpent-4-enyl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol |
| 1-NAPHTHALENEPROPANOL, alpha-ETHENYLDECAHYDRO-2-HYDROXY-alpha,2,5,5,8A-PENTAMETHYL-, (1R-(1alpha(R*),2beta,4Abeta,8Aalpha))- |
| 1-Naphthalenepropanol, alpha-ethenyldecahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (alphaR,1R,2R,4aS,8aS)-: (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol |
|
There are more than 10 synonyms. If you wish to see them all click here.
|