| 92-13-7 |
| Pilokarpin |
| Pilocarpol |
| Syncarpine |
| Pilocarpin |
| (+)-Pilocarpine |
| Ocusert pilo |
| Spersacarpine |
| Pilokarpol |
| Ocusert pilo-20 |
| Ocusert Pilo-40 |
| Ocusert P 20 |
| ocucarpine |
| Isoptocarpine |
| Pilocarpine chloride |
| Pilocarpinum |
| HSDB 3163 |
| EINECS 202-128-4 |
| UNII-01MI4Q9DI3 |
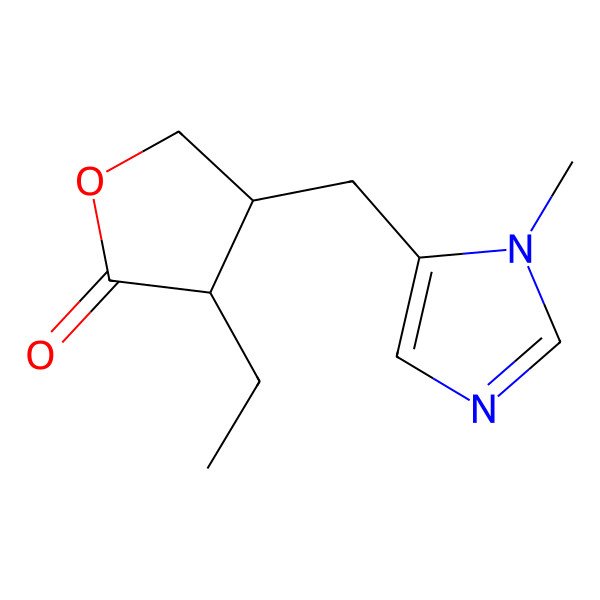
| (3S,4R)-3-Ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one |
| (3S,4R)-3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]dihydrofuran-2(3H)-one |
| 01MI4Q9DI3 |
| CHEBI:8207 |
| DTXSID1021162 |
| AI3-50523 |
| CHEMBL550 |
| (3S-cis)-3-Ethyldihydro-4-((1-methyl-1H-imidazol-5-yl)methyl)-2(3H)-furanone |
| Imidazole-5-butyric acid, alpha-ethyl-beta-(hydroxymethyl)-1-methyl-, gamma-lactone |
| Pilocarpine [USP:BAN:JAN] |
| DTXCID901162 |
| (3S,4R)-3-ethyl-4-[(3-methylimidazol-4-yl)methyl]oxolan-2-one |
| (3S,4R)-3-ethyldihydro-4-((1-methyl-1H-imidazol-5-yl)methyl)-2(3H)-furanone |
| (3S-cis)-3-ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl]-2(3H)-furanone |
| 2(3H)-Furanone, 3-ethyldihydro-4-((1-methyl-1H-imidazol-5-yl)methyl)-, (3S,4R)- |
| 92-13-7 (FREE BASE) |
| NCGC00023339-09 |
| 2(3H)-Furanone, 3-ethyldihydro-4-((1-methyl-1H-imidazol-5-yl)methyl)-, (3S-cis)- |
| Pilocarpine 100 microg/mL in Acetonitrile |
| (3S,4R)-3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]oxolan-2-one |
| beta-Pilocarpine hydrochloride |
| 2(3H)-Furanone, 3-ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl]-, (3S,4R)- |
| PILOCARPINE (MART.) |
| PILOCARPINE [MART.] |
| PILOCARPINE (USP-RS) |
| PILOCARPINE [USP-RS] |
| Pilocarpine (USP:BAN:JAN) |
| Pilocarpine nitrate salt |
| PILOCARPINE (USP MONOGRAPH) |
| PILOCARPINE [USP MONOGRAPH] |
| Pilocarpine, (+)- |
| CAS-92-13-7 |
| Pilocarpine (JAN/USP) |
| Ocusert pilo-20 (TN) |
| Pilocarpina |
| 3-Ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]dihydrofuran-2(3H)-one |
| Ocucarpine; Ocusert P 20; Ocusert Pilo |
| pilocarpine, Cytokine |
| Spectrum_001107 |
| Tocris-0694 |
| PILOCARPINE [MI] |
| Prestwick0_000449 |
| Prestwick1_000449 |
| Prestwick2_000449 |
| Prestwick3_000449 |
| Spectrum2_001284 |
| Spectrum3_000546 |
| Spectrum4_000478 |
| Spectrum5_001379 |
| PILOCARPINE [JAN] |
| PILOCARPINE [HSDB] |
| PILOCARPINE [VANDF] |
| PILOCARPINUM [HPUS] |
| Lopac0_000950 |
| Lopac0_000960 |
| SCHEMBL15146 |
| BSPBio_000498 |
| BSPBio_002191 |
| GTPL305 |
| KBioGR_000956 |
| KBioSS_001587 |
| PILOCARPINE [WHO-DD] |
| BIDD:GT0217 |
| DivK1c_000358 |
| SPBio_001287 |
| SPBio_002437 |
| BPBio1_000548 |
| CHEBI:39462 |
| HY-B0726A |
| KBio1_000358 |
| KBio2_001587 |
| KBio2_004155 |
| KBio2_006723 |
| KBio3_001691 |
| PILOCARPINE [ORANGE BOOK] |
| NINDS_000358 |
| HMS2089K17 |
| Tox21_110887 |
| BDBM50008072 |
| MFCD00153042 |
| (3S,4R)-3-ethyl-4-[(3-methylimidazol-4-yl)methyl]tetrahydrofuran-2-one |
| AKOS016010311 |
| Tox21_110887_1 |
| (3S-CIS)-3-ETHYLDIHYDRO-4- |
| CCG-205031 |
| DB01085 |
| SDCCGMLS-0003164.P005 |
| SDCCGSBI-0050924.P006 |
| IDI1_000358 |
| NCGC00023339-03 |
| NCGC00023339-06 |
| NCGC00023339-07 |
| NCGC00023339-08 |
| NCGC00023339-10 |
| NCGC00023339-11 |
| NCGC00023339-12 |
| NCGC00023339-13 |
| NCGC00023339-14 |
| NCGC00023339-16 |
| NCGC00023339-27 |
| NCGC00023339-28 |
| BS-18966 |
| NCI60_004403 |
| SBI-0050924.P004 |
| CS-0013746 |
| C07474 |
| D00525 |
| E87145 |
| AB00053525-27 |
| AB00053525_28 |
| AB00053525_29 |
| EN300-19632236 |
| Q411461 |
| BRD-K85090592-008-05-2 |
| BRD-K85090592-008-15-1 |
| SR-01000075339-11 |
| (3S,4R)-3-Ethyl-4-(1-methyl-1H-imidazol-5-ylmethyl)-4,5-dihydrofuran-2(3H)-one |
| rel-(3R,4S)-3-ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one |
| 102282-25-7 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|