| 16409-45-3 |
| 89-48-5 |
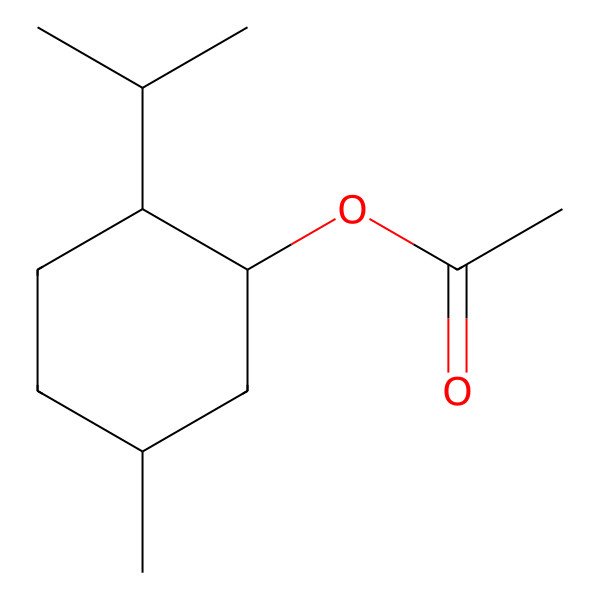
| Menthol, acetate |
| Menthyl acetate racemic |
| 29066-34-0 |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate |
| 2230-87-7 |
| FEMA Number 2668 |
| Neomenthyl acetate |
| (5-methyl-2-propan-2-ylcyclohexyl) acetate |
| 2623-23-6 |
| HSDB 824 |
| EINECS 240-459-6 |
| 5-Methyl-2-(1-methylethyl)cyclohexanol acetate |
| 2-Isopropyl-5-methylcyclohexyl acetate |
| Acetic acid, p-menth-3-yl ester, dl- |
| AI3-36197 |
| (+/-)-Menthyl acetate |
| EINECS 218-767-7 |
| d-Neomenthyl acetate |
| (+)-Neomenthyl acetate |
| l-Menthyl acetate (natural) |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R,2S,5R)-rel- |
| FEMA No. 2668 |
| Neomenthol acetate |
| NSC 3722 |
| EINECS 220-076-0 |
| NSC 52970 |
| L - menthyl acetate |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R,2R,5R)-rel- |
| L-Menthyl acetate;(-)-Menthyl acetate |
| BRN 2208505 |
| (+-)-Menthol acetate |
| (+-)-Menthyl acetate |
| Menthol, acetate, (1R,3R,4S)-(-)- |
| UNII-LF3LEI45OH |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R,2S,5R)- |
| ACETIC ACID, p-MENTH-3-YL ESTER, l- |
| (.+/-.)-Menthol acetate |
| Cyclohexan-1-ol, 2-isopropyl-5-methyl-, acetate, L- |
| enthyl acetate |
| Menthol, neo- |
| CYCLOHEXANOL, 5-METHYL-2-(1-METHYLETHYL)-, ACETATE, (1.ALPHA.,2.BETA.,5.ALPHA.)- |
| 20777-36-0 |
| l-Menthyl acetate (1alpha,2beta,5alpha) |
| (5R)-2-Isopropyl-5-methylcyclohexyl acetate |
| EC 201-911-8 |
| starbld0011430 |
| Menthyl acetate, 97% |
| MENTHYLACETATE97 |
| (2-isopropyl-5-methyl-cyclohexyl) acetate |
| acetic acid menthyl ester |
| SCHEMBL57396 |
| Menthol, acetate, (.+.)- |
| CHEMBL254585 |
| 2-Isopropyl-5-methylcyclohexyl acetate, (1.alpha.,2.beta.,5.alpha.)- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, 1-acetate, (1R,2R,5R)-rel- |
| DTXSID20859153 |
| NSC7350 |
| Acetic acid, p-menth-3-yl ester |
| CHEBI:191550 |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R-(1alpha,2beta,5alpha))- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1theta-(1alpha,2beta,5alpha))- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, [1R-(1.alpha.,2.beta.,5.alpha.)]- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, [1S-(1.alpha.,2.alpha.,5.beta.)]- |
| (1S)-(+)-MENTHYLACETATE |
| NSC-7350 |
| DL-Menthyl acetate, >=97%, FG |
| L-enthyl acetate;(-)-enthyl acetate |
| NSC122112 |
| AKOS015837885 |
| cis-1,3,trans-1,4- menthol acetate |
| ( .PLUS/MINUS.) - menthyl acetate |
| Menthyl acetate (1alpha,2beta,5alpha) |
| NSC 122112 |
| NSC-122112 |
| SB44841 |
| SB46743 |
| Menthol, acetate, cis-1,3,cis-1,4- |
| AS-83629 |
| FT-0604526 |
| FT-0604554 |
| FT-0628207 |
| FT-0631427 |
| FT-0690234 |
| FT-0694110 |
| W-100373 |
| Cyclohexanol, acetate, (1.alpha.,2.alpha.,5.beta.)- |
| Menthyl acetate, primary pharmaceutical reference standard |
| Cyclohexanol, acetate, [1S-(1.alpha.,2.alpha.,5.beta.)]- |
| Ciclohexanol, 5-metil-2-(1-metiletil)-, 1-acetato, (1r, 2s, 5r)-rel- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha.,2.alpha.,5.alpha.)- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha.,2.beta.,5.alpha.)-(.+.)- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1alpha,2alpha,5beta)- |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha.,2.beta.,5.alpha.)-(.+/-.)- |
| InChI=1/C12H22O2/c1-8(2)11-6-5-9(3)7-12(11)14-10(4)13/h8-9,11-12H,5-7H2,1-4H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|