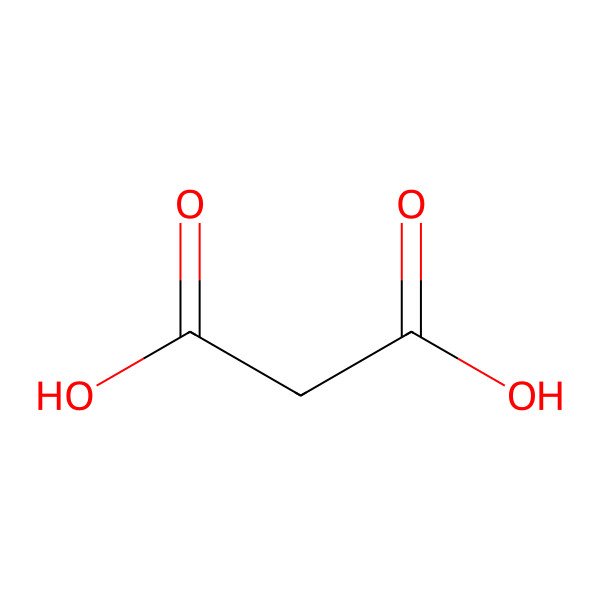
| propanedioic acid |
| 141-82-2 |
| Dicarboxymethane |
| Carboxyacetic acid |
| Methanedicarboxylic acid |
| Kyselina malonova |
| USAF EK-695 |
| 1,3-Propanedioic acid |
| Dicarboxylate |
| Malonicacid |
| Dicarboxylic acid |
| Kyselina malonova [Czech] |
| NSC 8124 |
| UNII-9KX7ZMG0MK |
| 9KX7ZMG0MK |
| AI3-15375 |
| H2malo |
| EINECS 205-503-0 |
| MFCD00002707 |
| BRN 1751370 |
| C3H4O4 |
| Methanedicarbonic acid |
| CHEBI:30794 |
| Thallium malonate |
| HOOC-CH2-COOH |
| NSC-8124 |
| Propane-1,3-dioic acid |
| alpha,omega-Dicarboxylic acid |
| DTXSID7021659 |
| HSDB 8437 |
| NSC8124 |
| 4-02-00-01874 (Beilstein Handbook Reference) |
| 1,3-Propanoic acid |
| PROPANEDIOLIC ACID |
| METAHNEDICARBOXYLIC ACID |
| 2fah |
| MLI |
| Malonic acid, 99% |
| Malonic acid (8CI) |
| 1o4m |
| Malonate dicarboxylic acid |
| Malonic acid, 99.5% |
| Propanedioic acid (9CI) |
| SCHEMBL336 |
| WLN: QV1VQ |
| D08JNG |
| MALONIC ACID [MI] |
| CH2(COOH)2 |
| CHEMBL7942 |
| MALONIC ACID [INCI] |
| DTXCID401659 |
| SCHEMBL1471092 |
| BDBM14673 |
| Propanedioic acid dithallium salt |
| Malonic acid, analytical standard |
| AMY11201 |
| BCP05571 |
| Malonic acid; (Carboxyacetic acid) |
| STR00614 |
| Tox21_200534 |
| AC8295 |
| LMFA01170041 |
| LS-832 |
| s3029 |
| STL194278 |
| Malonic acid, ReagentPlus(R), 99% |
| AKOS000119034 |
| CS-W019962 |
| DB02175 |
| PROPANEDIOIC ACID MALONIC ACID |
| NCGC00248681-01 |
| NCGC00258088-01 |
| BP-11453 |
| CAS-141-82-2 |
| SY001875 |
| Malonic acid, SAJ first grade, >=99.0% |
| FT-0628127 |
| FT-0628128 |
| FT-0690260 |
| FT-0693474 |
| M0028 |
| EN300-18457 |
| Malonic acid, Vetec(TM) reagent grade, 98% |
| C00383 |
| C02028 |
| C04025 |
| Q421972 |
| J-521669 |
| Z57965450 |
| F1908-0177 |
| Malonic acid, certified reference material, TraceCERT(R) |
| 592A9849-68C3-4635-AA3D-CBC44965EA3A |
| Malonic acid, sublimed grade, >=99.95% trace metals basis |
| DICARBOXYLIC ACID C3; PROPANEDIOLIC ACID; METHANEDICARBOXYLIC ACID |
| InChI=1/C3H4O4/c4-2(5)1-3(6)7/h1H2,(H,4,5)(H,6,7 |
| Malonic acid, anhydrous, free-flowing, Redi-Dri(TM), ReagentPlus(R), 99% |
| LML |
|
There are more than 10 synonyms. If you wish to see them all click here.
|