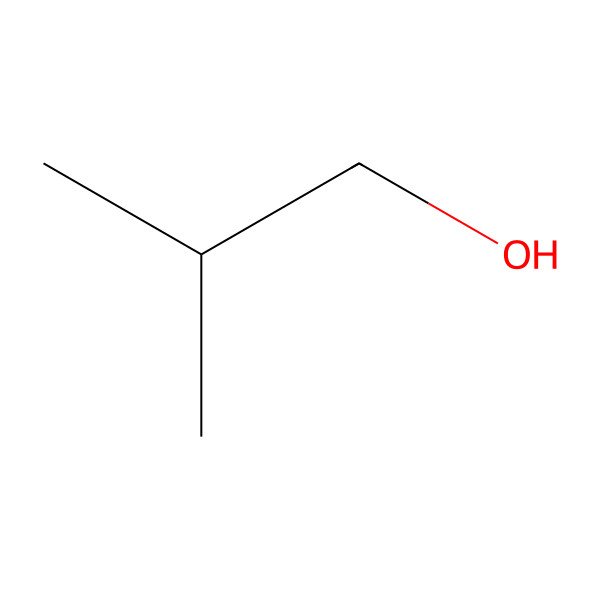
| 2-Methyl-1-propanol |
| ISOBUTYL ALCOHOL |
| 2-Methylpropan-1-ol |
| 78-83-1 |
| 1-Propanol, 2-methyl- |
| 1-Hydroxymethylpropane |
| Isopropylcarbinol |
| Iso-butyl alcohol |
| 2-Methylpropyl alcohol |
| i-Butyl alcohol |
| Isobutylalkohol |
| 2-Methylpropanol |
| Alcool isobutylique |
| 2-Methyl propanol |
| Fermentation butyl alcohol |
| i-Butanol |
| RCRA waste number U140 |
| 2-Methylpropanol-1 |
| FEMA No. 2179 |
| Isobutylalkohol [Czech] |
| FEMA Number 2179 |
| Isobutyl alcohol (natural) |
| Isopropyl carbinol |
| NSC 5708 |
| Alcool isobutylique [French] |
| HSDB 49 |
| CCRIS 2300 |
| Methyl-2 propanol-1 |
| iso-C4H9OH |
| EINECS 201-148-0 |
| MFCD00004740 |
| UN1212 |
| RCRA waste no. U140 |
| BRN 1730878 |
| UNII-56F9Z98TEM |
| 2-methyl-1-propanyl alcohol |
| AI3-01777 |
| 56F9Z98TEM |
| DTXSID0021759 |
| CHEBI:46645 |
| 2-Methyl-d3-propyl--d4 Alcohol |
| NSC-5708 |
| EC 201-148-0 |
| 4-01-00-01588 (Beilstein Handbook Reference) |
| iso-butanol |
| 68989-27-5 |
| isobutylalcohol |
| iso butanol |
| Butanol-iso |
| 2-methylpropanoI |
| iBuOH |
| Butanol (iso) |
| 2-methyl-propanol |
| iso-BuOH |
| i-BuOH |
| 2-methyl-l-propanol |
| 2-methyl-n-propanol |
| 2-metil-1-propanol |
| Isobutanol ACS grade |
| Propanol, 2-methyl- |
| alcohol 2-metilpropilo |
| 2-methyl-propan-1-ol |
| IAL (CHRIS Code) |
| Isobutanol, HPLC Grade |
| 1-propanol, 2-metil- |
| Isobutyl alcohol (8CI) |
| Isobutanol, Isobutyl alcohol |
| Isobutyl Alcohol Reagent ACS |
| BIDD:ER0628 |
| Isobutanol or isobutyl alcohol |
| ISOBUTYL ALCOHOL [II] |
| ISOBUTYL ALCOHOL [MI] |
| 2-Methyl-1-propanol, 99% |
| ISOBUTYL ALCOHOL [FCC] |
| NATURAL ISOBUTYL ALCOHOL |
| CHEMBL269630 |
| DTXCID601759 |
| 2 - methylpropan - 1 - ol |
| ISOBUTYL ALCOHOL [FHFI] |
| ISOBUTYL ALCOHOL [HSDB] |
| WLN: Q1Y1&1 |
| ISOBUTYL ALCOHOL [MART.] |
| NSC5708 |
| 2-Methyl-1-propanol, 99.5% |
| Isobutyl Alcohol (Fragrance Grade) |
| 2-Methyl-1-propanol, AR, 99% |
| Isobutanol, Spectrophotometric Grade |
| EINECS 273-552-5 |
| Tox21_201214 |
| ISOBUTYL ALCOHOL (ISOBUTANOL) |
| LMFA05000100 |
| NA1212 |
| STL185664 |
| 2-methyl-1-propanol(isobutyl alcohol) |
| 2-Methyl-1-propanol, LR, >=99% |
| AKOS000118740 |
| Isobutyl alcohol, >=99%, FCC, FG |
| LS-1756 |
| UN 1212 |
| 2-METHYL-1-PROPANOL [USP-RS] |
| CAS-78-83-1 |
| NCGC00091851-01 |
| NCGC00091851-02 |
| NCGC00258766-01 |
| 2-Methyl-1-propanol, analytical standard |
| 2-Methyl-1-propanol, anhydrous, 99.5% |
| 2-Methyl-1-propanol, for HPLC, 99.5% |
| Isobutyl alcohol, ACS reagent, >=99.0% |
| FT-0627343 |
| I0094 |
| EN300-19336 |
| Isobutyl alcohol 5000 microg/mL in Methanol |
| 2-Methyl-1-propanol 10 microg/mL in Methanol |
| Isobutyl alcohol, natural, >=99%, FCC, FG |
| 2-Methyl-1-propanol, ACS reagent, >=99.0% |
| Q151797 |
| 2-Methyl-1-propanol, p.a., ACS reagent, 99.0% |
| 2-Methyl-1-propanol, SAJ first grade, >=99.0% |
| J-509912 |
| 2-Methyl-1-propanol, JIS special grade, >=99.0% |
| F0001-2058 |
| InChI=1/C4H10O/c1-4(2)3-5/h4-5H,3H2,1-2H |
| 2-Methyl-1-propanol, ACS spectrophotometric grade, >=99.0% |
| 2-Methyl-1-propanol, reag. ISO, 99%, UV HPLC spectroscopic |
| Isobutanol or isobutyl alcohol [UN1212] [Flammable liquid] |
| Isobutanol or isobutyl alcohol [UN1212] [Flammable liquid] |
| 2-Methyl-1-propanol, puriss. p.a., ACS reagent, >=99.5% (GC) |
| 2-Methyl-1-propanol, BioUltra, for molecular biology, >=99.5% (GC) |
| 2-Methyl-1-propanol, United States Pharmacopeia (USP) Reference Standard |
| 2-Methyl-1-propanol, puriss. p.a., ACS reagent, reag. Ph. Eur., >=99% (GC) |
| 5OZ |
|
There are more than 10 synonyms. If you wish to see them all click here.
|