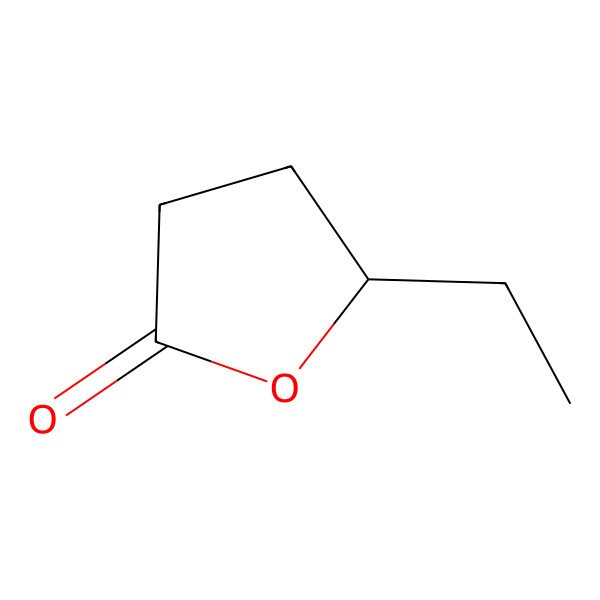
| 695-06-7 |
| gamma-Hexalactone |
| gamma-Hexanolactone |
| 4-Hexanolide |
| 6-Caprolactone |
| 5-Ethyldihydrofuran-2(3H)-one |
| 5-ethyloxolan-2-one |
| Tonkalide |
| 2(3H)-Furanone, 5-ethyldihydro- |
| Hexanolide-1,4 |
| Toukalide |
| Hexan-4-olide |
| 4-Ethyl-4-butanolide |
| 4-Hydroxyhexanoic acid lactone |
| gamma-Ethylbutyrolactone |
| 5-Ethyltetrahydro-2-furanone |
| .gamma.-Caprolactone |
| gamma-Ethyl-n-butyrolactone |
| 5-ETHYLDIHYDRO-2(3H)-FURANONE |
| .gamma.-Hexalactone |
| FEMA No. 2556 |
| Frutinal |
| .gamma.-Ethylbutyrolactone |
| NSC 24255 |
| NSC 134769 |
| .gamma.-Hexanolactone |
| Dihydro-5-ethyl-2(3H)-furanone |
| .gamma.-Ethyl-.gamma.-butyrolactone |
| EINECS 211-778-8 |
| MFCD00005401 |
| 4-Ethyl-4-hydroxybutanoic acid lactone |
| BRN 0107260 |
| UNII-J16NAT1G41 |
| Hexanoic acid, 4-hydroxy-, gamma-lactone |
| AI3-36655 |
| CHEMBL192458 |
| J16NAT1G41 |
| DTXSID8041298 |
| CHEBI:85235 |
| NSC-24255 |
| NSC-134769 |
| 5-17-09-00040 (Beilstein Handbook Reference) |
| Hexanoic acid, .gamma.-lactone |
| |A-Caprolactone |
| -caprolactone |
| -Hexalactone |
| gamma-Ethyl-gamma-butyrolactone |
| Gamma Hexalactone |
| 4-Ethylbutanolide |
| hexa-4-olide |
| 4-Hydroxyhexanoate |
| gamma -Caprolactone |
| 4-hydroxy-Hexanoate |
| hexan - 4 - olide |
| starbld0016506 |
| .GAMMA.-LACTONE |
| gamma-Caprolactone, 98% |
| SCHEMBL36360 |
| .GAMMA.-CAPROLACTONE- |
| .gamma.-Ethyl-n-butyrolactone |
| 5-Ethyl-dihydro-furan-2-one |
| 4-hydroxy-Hexanoic acid lactone |
| GAMMA-HEXALACTONE [FCC] |
| DTXCID6021298 |
| GAMMA-CAPROLACTONE [INCI] |
| .GAMMA.-HEXALACTONE [FHFI] |
| 5-Ethyldihydro-2(3H)-furanone # |
| NSC24255 |
| Hexanoic acid, 4-hydroxy-, lactone |
| Tox21_300875 |
| (+/-)-4-ETHYLBUTYROLACTONE |
| AC2069 |
| BDBM50167994 |
| LMFA07040010 |
| NSC134769 |
| s6265 |
| (+/-)-.GAMMA.-HEXALACTONE |
| 4-ethylbutanolide (gamma-hexalactone) |
| 4-hydroxy-Hexanoic acid gamma-lactone |
| gamma-Hexalactone, analytical standard |
| AKOS015908187 |
| CS-W016608 |
| DS-6470 |
| gamma-Hexalactone, natural, 97%, FG |
| HY-W015892 |
| LS-2794 |
| SB47678 |
| gamma-Hexalactone, >=98%, FCC, FG |
| NCGC00248198-01 |
| NCGC00254779-01 |
| CAS-695-06-7 |
| SY015382 |
| FT-0626614 |
| FT-0701250 |
| H0514 |
| Hexanoic acid, 4-hydroxy-, .gamma.-lactone |
| EN300-156820 |
| gamma-Caprolactone, Vetec(TM) reagent grade, 98% |
| (+/-)-.GAMMA.-ETHYL-.GAMMA.-BUTYROLACTONE |
| gamma-Ethyl-gamma-butyrolactone, gamma-Caprolactone |
| Q27158424 |
| Z1255423463 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|