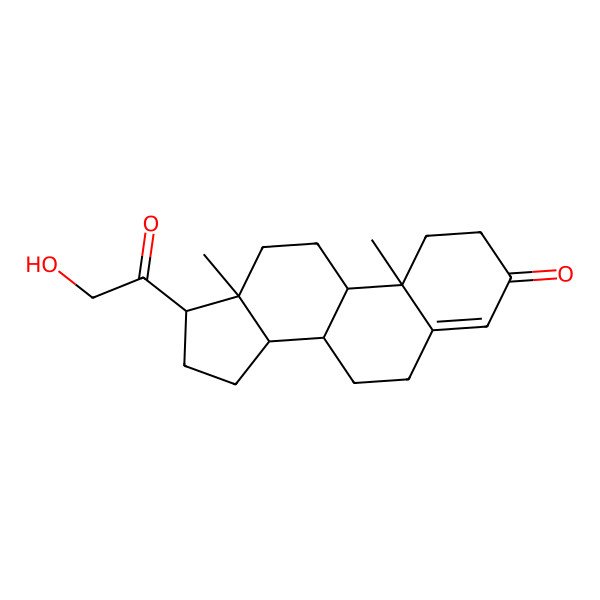
| desoxycorticosterone |
| 11-Deoxycorticosterone |
| Deoxycorticosterone |
| 21-Hydroxyprogesterone |
| Cortexone |
| 64-85-7 |
| Deoxycortone |
| 11-Desoxycorticosterone |
| Reichstein's substance Q |
| 4-Pregnen-21-ol-3,20-dione |
| Desossicortone |
| Desoxicortonum |
| Desoxycorticosteronum |
| Kendall's desoxy compound B |
| 11-Dehydroxycorticosterone |
| Corticosterone, 11-deoxy- |
| 21-Hydroxy-4-pregnene-3,20-dione |
| Progesterone, 21-hydroxy- |
| 21-HYDROXYPREGN-4-ENE-3,20-DIONE |
| Pregn-4-ene-3,20-dione, 21-hydroxy- |
| 1,2(3H)-Deoxycorticosterone |
| Desoxicortona |
| Desoxycortonum |
| DOC |
| NSC 11319 |
| 11-Deoxy Corticosterone |
| Desoxycortonum [INN-Latin] |
| EINECS 200-596-4 |
| Desoxicortona [INN-Spanish] |
| UNII-40GP35YQ49 |
| DTXSID0045254 |
| CHEBI:16973 |
| 40GP35YQ49 |
| 21-hydroxy-Pregn-4-ene-3,20-dione |
| MLS000028537 |
| MLS001076270 |
| DTXCID8025254 |
| 21-Hydroxy-3,20-dioxopregn-4-ene |
| Desoxycortone (INN) |
| 21-Hydroxy-Delta4-pregnene-3,20-dione |
| SMR000058340 |
| DESOXYCORTONE [INN] |
| Desoxycortonum (INN-Latin) |
| Desoxicortona (INN-Spanish) |
| DESOXYCORTONE (MART.) |
| DESOXYCORTONE [MART.] |
| Desossicortone [DCIT] |
| [4-14C]-11-deoxycorticosterone |
| NSC-11319 |
| 1CA |
| 11 Decorticosterone |
| 11-Decorticosterone |
| 21 Hydroxyprogesterone |
| 11-deoxy-Corticosterone |
| Desoxycortone [INN:BAN] |
| Desoxycorticosteron |
| 'Reichstein Q' |
| CAS-64-85-7 |
| NCGC00016292-01 |
| 11-Dcortic |
| 21 Hydroxy 4 pregnene 3,20 dione |
| DOCA (Salt/Mix) |
| acAtate de dAsoxycortone |
| Opera_ID_581 |
| 21-hydroxy-Progesterone |
| Prestwick0_000957 |
| Prestwick1_000957 |
| Prestwick2_000957 |
| Prestwick3_000957 |
| bmse000535 |
| SCHEMBL4065 |
| CHEMBL1498 |
| BSPBio_000954 |
| SPBio_003103 |
| DESOXYCORTONE [WHO-DD] |
| BDBM8582 |
| BPBio1_001050 |
| GTPL2871 |
| DEOXYCORTICOSTERONE [MI] |
| hydroxy-4-pregnene-3,20-dione |
| H02AA02 |
| 4-pregnene-3,20-dione-21-ol |
| HMS1570P16 |
| HMS2097P16 |
| HMS2235G07 |
| HMS3714P16 |
| D4-Pregnene-21-ol-3,20-dione |
| DESOXYCORTICOSTERONE [VANDF] |
| Tox21_110356 |
| Tox21_302402 |
| CMC_13409 |
| LMST02030087 |
| Delta4-Pregnene-21-ol-3,20-dione |
| 21-Hydroxy-D4-pregnane-3,20-dione |
| 21-Hydroxy-D4-pregnene-3,20-dione |
| AKOS005111365 |
| Tox21_110356_1 |
| CCG-220957 |
| 21-Hydroxypregn-4-ene-3,20-dione # |
| NCGC00021304-03 |
| NCGC00021304-05 |
| NCGC00256184-01 |
| (8S,9S,10R,13S,14S,17S)-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one |
| 21-Hydroxy-Delta4-pregnane-3,20-dione |
| 21-Hydroxyprogesterone, >=97% (HPLC) |
| AS-77189 |
| HY-113414 |
| AB00490029 |
| CS-0059402 |
| (14beta)-21-hydroxypregn-4-ene-3,20-dione |
| C03205 |
| D07792 |
| Q948846 |
| SR-01000003106 |
| 21-HYDROXY-.DELTA.4-PREGNENE-3,20-DIONE |
| SR-01000003106-2 |
| W-104814 |
| BRD-K98521173-001-03-8 |
| BD5D7BC9-0CD8-404E-9BA0-F670962012F8 |
| 4-PREGNEN-21-OL-3,20-DIONE; DOC; 21-HYDROXYPROGESTERONE |
| (1S,2R,10S,11S,14S,15S)-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadec-6-en-5-one |
| (8S,10R,13S,17S)-17-(2-Hydroxy-acetyl)-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one |
|
There are more than 10 synonyms. If you wish to see them all click here.
|