| 490-23-3 |
| epsilon-Tocopherol |
| Tocotrienol, beta |
| a-tocotrienol |
| D-beta-Tocotrienol |
| .epsilon.-Tocopherol |
| UNII-CHH810ZM8C |
| CHH810ZM8C |
| EINECS 207-708-0 |
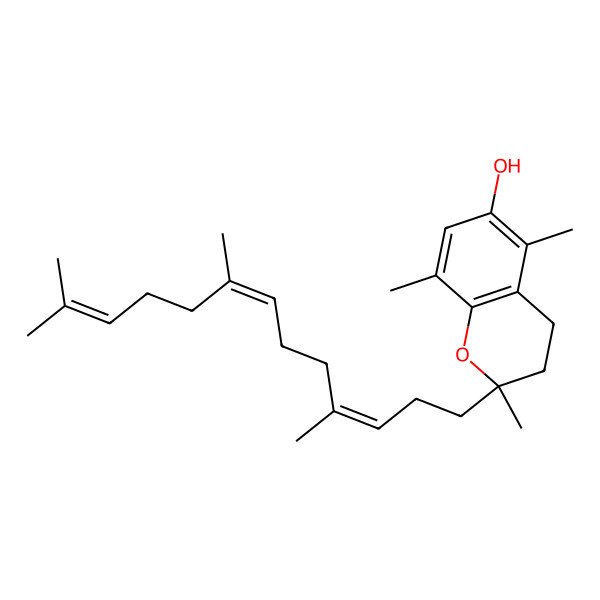
| (2R)-2,5,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl]-3,4-dihydrochromen-6-ol |
| 2,5,8-Trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl]-3,4-dihydrochromen-6-ol |
| (2R)-3,4-dihydro-2,5,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-2H-1-benzopyran-6-ol |
| 3,4-Dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyl-trideca-3,7,11-trienyl)-2H-1-benzopyran-6-ol |
| epsilon-Tokoferol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-((3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl)-, (2R)- |
| 2H-1-benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-, (2R)- |
| 2R,5,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl]-3,4-dihydro-2H-chromen-6-ol |
| beta -Tocotrienol |
| 2H-1-BENZOPYRAN-6-OL, 3,4-DIHYDRO-2,5,8-TRIMETHYL-2-(4,8,12-TRIMETHYL-3,7,11-TRIDECATRIENYL)-, (R-(E,E))- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-, [R-(E,E)]- |
| D-beta- Tocotrienol |
| .BETA.-TOCOTRIENOL |
| .EPSILON.-TOKOFEROL |
| Tocotrienol, 5,8-dimethyl |
| BIDD:PXR0058 |
| SCHEMBL66599 |
| D-.BETA.-TOCOTRIENOL |
| 2,5,8-trimethyl-2-(4,8,12-trimethyltrideca-3,7,11-trienyl)chroman-6-ol |
| SCHEMBL16430159 |
| CHEBI:33275 |
| .BETA.-TOCOTRIENOL [MI] |
| DTXSID50883401 |
| BETA-TOCOTRIENOL [WHO-DD] |
| beta-Tocotrienol, analytical standard |
| LMPR02020055 |
| AKOS040744870 |
| [R-(E,E)]-3,4-Dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)-2H-1-benzopyran-6-ol |
| J37.066E |
| MS-27089 |
| HY-108693 |
| CS-0029986 |
| A934798 |
| Q171542 |
| 6-Chromanol,2,5,8-trimethyl-2-(4,8,12-trimethyl-3,7,11-tridecatrienyl)- (8CI) |
| (2R)-2,5,8-Trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatr ien-1-yl]-6-chromanol |
| (2R)-2,5,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl]-3,4-dihydro-2H-chromen-6-ol |
| (2R)-2beta,5,8-Trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-3,4-dihydro-2H-1-benzopyran-6-ol |
| 2H-1-BENZOPYRAN-6-OL, 3,4-DIHYDRO-2,5,8-TRIMETHYL-2- ((3E,7E)-4,8,12-TRIMETHYL-3,7,11-TRIDECATRIEN-1-YL)-, (2R)- |
| 2H-1-BENZOPYRAN-6-OL, 3,4-DIHYDRO-2,5,8-TRIMETHYL-2- ((3E,7E)-4,8,12-TRIMETHYL-3,7,11-TRIDECATRIENYL)-, (2R)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-((3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrien-1-yl)-, (2R)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-[(3E,7E)-4,8,12-trimethyl-3,7,11-tridecatrienyl]-, (2R)- (9CI) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|